A 3D lung model is essentially a digital, patient-specific copy of the respiratory system, built from medical images like CT scans. It's far more than a static picture; think of it as an interactive, explorable map of a person's unique lungs, airways, and blood vessels. This shift from flat, 2D images to dynamic, three-dimensional tools is fundamentally changing medicine.
What Is a 3D Lung Model and Why Is It a Game Changer?

For decades, doctors have depended on flat images from CT scans and X-rays to figure out what’s wrong with our lungs. Imagine a standard CT scan as a paper road map. It’s useful, sure—it shows you the main roads and landmarks. But it can't show you the hills, valleys, or the actual feel of the terrain. That's the core limitation of a two-dimensional view.
A 3D lung model, on the other hand, is like having a fully interactive, high-definition globe of that same territory. It’s not just a picture; it’s a true digital twin of the patient’s respiratory system. This virtual replica is created by taking hundreds of individual 2D scan slices and using smart software to piece them together into a complete, volumetric model.
From Flat Images to Interactive Anatomy
Making the jump from 2D to 3D is a much bigger deal than just a visual enhancement. It gives medical teams a dynamic tool they can spin around, zoom in on, and virtually slice through from any angle. Suddenly, they can see the precise spatial relationship between a tumor, a major artery, and the nearest airway—all within the context of one person's unique body.
A 3D model takes abstract data points from a scan and turns them into a tangible, intuitive representation of human anatomy. This opens the door for preoperative planning and patient conversations that were simply out of reach with flat images.
The impact is massive. A diagnostic image is no longer just for diagnosis; it becomes a critical asset for planning and carrying out treatment.
Why This Technology Matters
The true power of a 3D lung model comes alive in its real-world applications, which are rewriting the playbook for managing lung diseases. It introduces a level of detail that helps medicine move from one-size-fits-all approaches to genuinely personalized care.
Here’s where it makes a difference:
- Surgical Precision: Surgeons can run through an operation virtually before ever making an incision. They can map out the safest route to remove a tumor while saving as much healthy lung tissue as possible. This often means shorter surgeries and better results for the patient.
- Smarter Drug Development: For conditions like asthma or COPD, researchers can use these models to simulate airflow and see how an inhaled medication might actually travel through a specific patient's airway tree.
- Improved Patient Understanding: When a patient can see a visual, interactive model of their own lungs, their condition and the proposed treatment make so much more sense. This empowers them to be a more active participant in their own care.
At its core, the 3D lung model is a foundational tool. It provides the detailed anatomical blueprint required for more accurate surgical navigation systems, customized therapies, and even the 3D printing of physical organ models for training. It’s setting an entirely new standard for respiratory medicine.
How a Digital Twin of the Lungs Is Created
So, how do we get from a series of flat, grayscale medical images to a dynamic, interactive 3D lung model? It's a bit like a digital sculptor carefully carving away stone to reveal the intricate statue hidden inside. The process doesn’t start with fancy software, but with the raw data itself—the patient's scans.
The entire journey from a 2D scan to a 3D digital twin lives or dies by the quality of that initial data. The foundation for this work is almost always a high-resolution computed tomography (CT) scan, which gives us hundreds, sometimes thousands, of detailed cross-sectional images of the chest.
Acquiring the Foundational Data
A CT scanner works by taking a rapid succession of X-ray images from different angles all around the body. A computer then processes these images to create detailed "slices." For a lung model to be truly useful, these slices need to be incredibly thin—often less than a millimeter—to pick up on the fine details, like the tiniest blood vessels and the delicate, branching airways.
This first step is all about data collection. What you end up with is a huge dataset in the standard DICOM (Digital Imaging and Communications in Medicine) format. It's packed with information, but at this stage, it's still just a stack of 2D pictures, not a unified 3D structure.
The Critical Art of Segmentation
Next up is the most crucial, and often the most challenging, part of the process: segmentation. Think of this as the digital equivalent of meticulously tracing every important structure on each individual CT slice. The goal here is to isolate and label the different anatomical parts—the lung lobes, airways (like the trachea and bronchi), pulmonary arteries, veins, and of course, any abnormalities like tumors or nodules.
It’s a bit like trying to color in a highly complex coloring book where the lines are faint and occasionally blend into one another. This is where specialists, often working alongside sophisticated AI algorithms, have to painstakingly outline the boundaries of each tissue type.
The accuracy of the final 3D lung model is directly proportional to the precision of its segmentation. An error of just a few pixels at this stage could throw off a tumor's location relative to a major blood vessel, which could have serious clinical consequences.
This step is what separates the "signal" (the anatomy we care about) from the "noise" (all the surrounding tissue). It’s what gives the final model its anatomical intelligence.
Stacking Slices into a 3D Reconstruction
Once every structure has been neatly segmented across the entire stack of CT slices, the final step is 3D reconstruction. This is where specialized software takes all those digitally traced 2D layers and stacks them up, almost like building a layer cake.
The software then interpolates the data between each slice to create a smooth, continuous, and geometrically accurate volumetric model. The result is no longer just a collection of flat images but a single, navigable 3D object—the patient's digital lung twin. This model can be spun around, zoomed into, and even virtually dissected to expose complex internal relationships that are nearly impossible to see otherwise.
This workflow, from raw scan to a tangible model, is broken down into a few key stages.
Key Stages in Building a 3D Lung Model
| Stage | Primary Goal | Key Outcome |
|---|---|---|
| Data Acquisition | To capture high-resolution, cross-sectional images of the patient's lungs. | A complete set of DICOM-formatted CT scan slices. |
| Segmentation | To digitally trace and label all relevant anatomical structures on each 2D slice. | A collection of segmented images where anatomy is clearly defined. |
| 3D Reconstruction | To stack and interpolate the segmented slices into a cohesive, volumetric object. | A fully interactive and geometrically accurate 3D lung model. |
Each stage builds directly on the last, turning abstract data into a powerful clinical tool.
This infographic captures the streamlined workflow, from the initial scan all the way to the final, usable model.
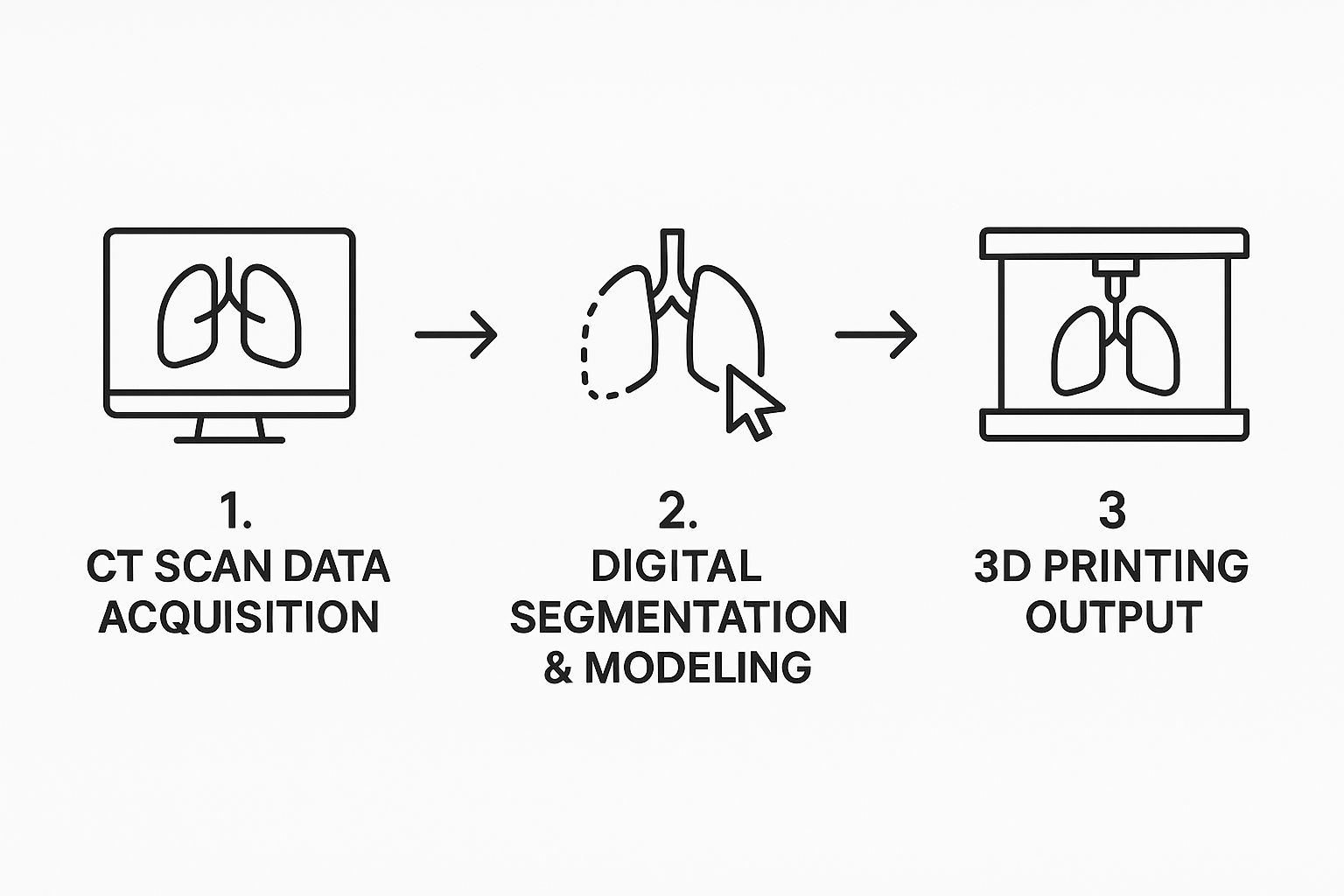
As the visualization shows, raw data is systematically refined through digital processing. It can even be used to create a physical object, completing the journey from pixels on a screen to a practical tool in a surgeon's hands.
How AI Gives 3D Lung Models Superpowers
Building a 3D lung model is a bit like sculpting. It's a detailed, meticulous process. But the biggest challenge, the one thing that keeps this technology from being used everywhere, is the bottleneck of manual segmentation.
Imagine asking a highly skilled radiologist to sit down and painstakingly trace every tiny airway, blood vessel, and nodule on hundreds of individual CT scan slices. It’s an enormous, time-consuming job. We're talking hours for a single patient, which just isn't practical in a busy clinic.
This is where Artificial Intelligence changes the game. AI isn't here to replace the expert; it's here to act as an incredibly efficient and tireless assistant. It takes the tedious, manual work of segmentation and transforms it into a fast, automated workflow.
Deep learning algorithms are especially good at this. You can think of them as pattern-recognition specialists. By showing them thousands of expertly annotated lung scans, they learn to see the subtle differences in texture and density that distinguish a bronchial tube from a blood vessel, or healthy tissue from a suspicious lesion.
Putting Segmentation on Autopilot
AI-powered tools dive directly into the raw DICOM data from a CT scan and automatically outline all the critical structures. Instead of a clinician manually clicking and drawing boundaries slice by slice, the algorithm can map out the entire lung volume in a matter of minutes. This drastically cuts down the time it takes to build a complete 3D lung model.
A popular algorithm for this kind of work is called a U-Net. Its unique design is fantastic at pixel-level classification, making it a perfect tool for pinpointing the exact location of anatomical features within a medical image.
This automated approach delivers some major wins:
- Speed: A task that used to take hours can now be done in minutes. Research from as far back as 2018 on lung cancer detection laid the foundation for systems that can chew through massive amounts of imaging data. That kind of speed is what makes 3D modeling practical for time-sensitive decisions.
- Consistency: Let's be honest, manual tracing can vary. Two different experts might produce slightly different outlines, and even the same expert might be less consistent at the end of a long day. An AI, on the other hand, applies the same logic every single time, giving you incredibly reproducible results.
- Precision: Because these models are trained on huge datasets, they often spot and segment things that are tough for the human eye to see, like the smallest nodules or the most delicate bronchial branches.
AI isn't just about making things faster. It's about achieving a level of detail and consistency that was simply out of reach at scale before. It elevates the quality of the model from the ground up.
This leap in automated precision is what’s finally unlocking the widespread, practical use of patient-specific models in everyday clinical care.
Better Diagnostics, Smarter Planning
The benefits of AI go way beyond just faster segmentation. The incredibly detailed models it helps create give clinicians richer information to work with, leading to better, more confident decisions.
With a precisely segmented 3D lung model on screen, a surgeon can see exactly how a tumor is situated next to critical blood vessels and airways. They can measure volumes with pinpoint accuracy, plan their surgical approach with much greater confidence, and see potential roadblocks before the first incision is ever made. Some studies have even found that surgeons change their initial operative plans after reviewing these detailed 3D reconstructions.
What's more, these AI-generated models are becoming the building blocks for other medical innovations. The rich data can be used to quickly train other specialized models—for example, one that predicts whether a nodule is malignant or one that classifies different types of lung disease. This creates a feedback loop that speeds up both research and the development of new diagnostic tools.
By taking on the heavy lifting of segmentation, AI gives clinicians back their most valuable resource: time. It allows them to focus on what they do best—interpreting complex data, strategizing treatments, and caring for their patients. It's this partnership between human expertise and machine efficiency that makes the modern 3D lung model such a powerful tool in medicine.
A New Era for Thoracic Surgery: Visualizing the Lungs in 3D
In the high-stakes world of thoracic surgery, precision isn't just a goal; it's a necessity. For decades, surgeons have planned intricate lung procedures by mentally piecing together flat, two-dimensional CT scans. Think of a mechanic trying to fix a modern engine using only a stack of black-and-white photos—it’s doable, but a lot is left to interpretation and guesswork.
This is precisely the challenge that a 3D lung model solves. It takes us from static, sliced images to a dynamic, interactive replica of a patient's unique anatomy. This isn't just a cosmetic upgrade; it's a fundamental shift in how surgeons prepare for and perform life-saving operations.
With a detailed 3D view, a surgeon can essentially "rehearse" an entire procedure before stepping into the operating room. They can rotate the model, zoom in on a suspicious nodule, and trace its exact relationship to critical structures like the pulmonary artery or the bronchial tree. This virtual walkthrough helps them spot potential pitfalls and map out the safest, most effective surgical path before making a single incision.
Planning with Unprecedented Clarity
The difference between planning with 2D scans and a 3D model is like navigating with a paper street map versus using a GPS with live traffic updates. The map shows you the roads, sure. But the GPS gives you the complete, real-time picture, warning you about roadblocks and suggesting the best detours.
This deeper anatomical insight is absolutely critical in lung cancer surgery. The surgeon's job is a delicate balance: remove all cancerous tissue while preserving as much healthy, functional lung as possible. A 3D lung model provides the detailed blueprint needed to make these vital judgment calls with a much higher level of confidence.
For surgeons, a patient-specific 3D lung model transforms preoperative planning from an act of interpretation into an act of direct visualization. It removes guesswork and replaces it with a clear, navigable anatomical road map.
This enhanced clarity leads directly to more precise and effective surgical strategies, which can make a world of difference for the patient.
Improving Surgical Precision and Outcomes
This technology is far from a novelty; it has a real, measurable impact on how surgery is performed. The detailed 3D visualization of a patient's specific anatomy—including their unique broncho-vascular structures and lung nodules—allows surgeons to more accurately determine the scope of the resection required. They can spot anatomical variations that might complicate the procedure, details often hidden or ambiguous in flat scans alone.
In fact, studies have shown that using a 3D lung model for planning can dramatically improve a surgeon's understanding. In some cases, it leads them to change their initial surgical plan entirely in favor of a safer or more effective approach. This detailed view helps them map out where to tie off blood vessels and airways with greater accuracy, preventing accidental damage to delicate structures—a key factor in minimizing complications. Learn more about how these models are enhancing lung cancer surgery and intraoperative navigation.
This ability to meticulously plan each step brings several direct benefits:
- Preserving Healthy Tissue: By clearly seeing a tumor's borders, surgeons can perform more targeted resections. This saves healthy lung tissue and can significantly improve a patient’s quality of life after surgery.
- Reducing Operative Time: When surgeons know exactly what to expect, they spend less time navigating surprises in the operating room, which can shorten the overall procedure time.
- Boosting Surgeon Confidence: A thorough virtual rehearsal gives the entire surgical team a shared mental model and a greater sense of confidence going into the operation.
Ultimately, bringing the 3D lung model into thoracic surgery is about tilting the odds in the patient's favor. It arms surgeons with the insight they need to perform incredibly complex procedures with greater accuracy, leading to safer operations and better long-term outcomes for people facing serious lung conditions.
Optimizing Inhalable Drugs and Respiratory Therapy
Beyond the operating room, 3D lung models are having a huge impact on how we develop new medicines and treat breathing problems. For the millions of people living with asthma or Chronic Obstructive Pulmonary Disease (COPD), getting the right dose of medication to the right spot in the lungs is everything.
The trouble is, traditional drug development often treats all lungs as if they're the same. But they aren't. Your airway anatomy is as unique as your fingerprint, with its own specific diameters and branching patterns. This is why a standard inhaler might be a lifesaver for one person and far less effective for another.
Simulating Airflow and Drug Delivery
This is where a patient-specific 3D lung model becomes a game-changer. Think of it as a digital twin of a person's lungs—a virtual playground for testing inhalable drugs. Researchers use these models to run incredibly detailed airflow simulations, a technique called computational fluid dynamics (CFD).
These simulations let them literally watch how air flows through an individual’s unique bronchial tree when they breathe in. By adding virtual drug particles to the simulation, scientists can track exactly where they go. Do they successfully navigate the complex maze of airways to reach the diseased tissue deep inside? Or do they just get stuck in the throat, where they do little good?
This virtual analysis helps answer mission-critical questions:
- Is the drug particle size just right for reaching the smallest airways?
- Does the inhaler's design create the perfect puff of aerosol?
- How does a person’s unique breathing style change where the medicine lands?
This approach flips the script on drug development. Instead of relying on broad averages, we can start fine-tuning treatments for individual patients. It’s like creating a virtual prototype of a new drug and delivery device before ever starting a costly clinical trial.
Personalizing Respiratory Medicine
The end game here is truly personalized respiratory therapy. Picture this: a doctor takes your CT scan, generates your 3D lung model, and uses it to prescribe the perfect inhaler and drug formula for you. It’s a completely data-driven approach designed for maximum impact.
This technology is also pushing the boundaries of research. For example, scientists at the University of Delaware are using 3D-printed models to mimic human breathing and see how aerosol drugs actually deposit in the lungs. This kind of work is vital because, for any respiratory medicine to work, it has to hit its target. You can read more about how this 3D lung model is advancing aerosol medication research.
By running countless simulations on different 3D lung models, pharmaceutical companies can design better products that work for a wider variety of people. This ultimately leads to more effective treatments, fewer side effects, and better lives for patients. It's a fantastic example of how a digital tool can solve a very real, very physical medical challenge.
From Digital Model to Physical Replica with 3D Printing
An interactive 3D lung model on a screen is incredibly powerful, but sometimes, you just need something you can hold. This is where 3D printing comes in, bridging the gap between digital data and the physical world. It transforms a complex dataset into a tangible, life-sized replica a surgeon can actually have in their hands.
Imagine a thoracic surgeon planning a tricky tumor removal. Instead of just spinning a model on a monitor, they can now pick up a physical copy of their patient's unique lung anatomy. They can feel the tumor's exact shape, trace the path of major blood vessels with their fingers, and rehearse the surgical plan. This tactile understanding provides a level of insight that a screen simply can't replicate.
A Powerful Tool for Surgical Planning and Training
These patient-specific models are a game-changer for preparing for operations on complex or unusually located tumors. Holding a physical replica lets the surgical team anticipate challenges and fine-tune their strategy long before the patient is even in the operating room. This is especially vital in thoracic oncology, where every anatomical detail matters.
For central lung cancers, 3D printing is a particularly promising step forward. One study involving 41 3D-printed lung models found they helped surgeons clearly visualize the patient's specific tumor and airway anatomy before the first incision. This improved perspective helped them decide between different surgical approaches and could potentially lower risks during the operation. You can learn more about how 3D printing is aiding thoracic oncology on pmc.ncbi.nlm.nih.gov.
Beyond the OR, these models are exceptional training tools. Medical residents and fellows can practice difficult procedures on realistic, patient-derived models instead of just relying on generic textbook diagrams. This kind of hands-on experience is invaluable for building skill and confidence in a safe, controlled setting.
A physical 3D lung model takes the abstract concept of a patient's anatomy and makes it concrete. For a surgeon, it’s a detailed rehearsal tool; for a trainee, it's an advanced educational aid; and for a patient, it’s a source of clarity and empowerment.
Empowering Patients Through Clear Communication
Finally, let's not forget the patient. Explaining a complex lung condition and the proposed surgery can be tough. But handing a patient a model of their own lung? That changes the entire conversation.
It allows them to see exactly where the problem is and understand the surgeon's plan in a way that’s immediately intuitive. This simple act can demystify the medical jargon, reduce anxiety, and empower patients to be more informed participants in their own care. The physical 3D lung model isn't just a piece of plastic; it's a powerful instrument for planning, training, and, most importantly, communication.
Common Questions About 3D Lung Models
As 3D lung modeling moves from the research lab into everyday clinical practice, a lot of practical questions pop up. It's one thing to see the technology in a presentation, but another to think about how it actually works day-to-day. Let's tackle some of the most common things people ask.
How Is Patient Data Kept Private and Secure?
This is, without a doubt, the most critical question. Patient privacy is non-negotiable. The medical data used for these models, usually CT scans in a format called DICOM, is protected by strict regulations like HIPAA in the United States.
Before that data ever touches a modeling software, it goes through a crucial step: de-identification or anonymization. Think of it like redacting a document—all personally identifiable information is scrubbed clean. Names, birthdates, medical record numbers, everything is removed. What’s left is pure anatomical data, completely disconnected from the individual, protecting their identity while still allowing us to build the model.
How Long Does It Take to Create a Model?
The answer to this has changed drastically in just the last few years, and the hero of the story is AI. Not long ago, a skilled technician would have to sit down and manually trace out the airways and vessels from a CT scan. This could easily take several hours for a single, complex case, which just isn't practical for most clinical situations.
Now, with AI-driven segmentation, that same job can be done in minutes. The total turnaround, from getting the CT scan to having a fully interactive 3D lung model ready for a surgeon to review, can be under an hour. That speed is what makes this a game-changer for time-sensitive decisions, like prepping for an urgent surgery.
The efficiency of AI has shifted the bottleneck from hours of manual labor to a streamlined, rapid process. This acceleration is critical for integrating 3D modeling into daily clinical workflows, making personalized anatomical insights accessible when they matter most.
Are These Models Expensive and Widely Available?
Both the cost and availability are getting better all the time. Initially, the powerful computers and specialized software needed were a huge barrier, limiting this technology to a handful of well-funded institutions. But as AI tools have matured and become more common, the costs have started to come down.
You'll now find this technology in many major medical centers, especially in departments focused on thoracic surgery and oncology where precision is everything. It's not yet a standard piece of equipment in every local clinic, but the clear benefits—better surgical planning, improved patient outcomes—are pushing it into the mainstream. The 3D lung model is quickly moving from a "nice-to-have" to a core part of advanced respiratory care.
At PYCAD, we build the AI engines that make these advanced medical tools possible. We have the deep expertise to handle, annotate, and process complex medical imaging data, helping you create and deploy the next generation of diagnostic and planning solutions. Discover how our AI services can accelerate your medical imaging projects by visiting the PYCAD website.