Why Statistical Shape Modeling Matters in Modern Medicine
Imagine you're a doctor, trying to spot the earliest whispers of a disease in a patient's scan. You're looking for subtle changes, the kind that only years of experience can teach you to see. Now, imagine giving a computer that same expert eye. That's the promise of statistical shape modeling (SSM).
This powerful technique takes thousands of medical images and transforms them into mathematical representations of anatomical structures, essentially creating a fingerprint system for the human body. Each heart, lung, or bone has its own unique shape signature, and SSM lets us analyze these signatures to uncover hidden patterns related to health and disease.
It's like moving from a simple ruler to a high-precision 3D scanner. We can go beyond basic measurements like volume and size and dive into the intricacies of shape. Think about the human heart: SSM allows us to analyze its precise contours, detecting subtle deviations that might hint at early-stage heart disease, long before traditional methods would catch it.
This ability to quantify shape makes SSM incredibly valuable for predicting disease progression and personalizing treatment plans.

The image above, from the Wikipedia page on statistical shape analysis, shows a series of hand outlines. This illustrates how SSM can represent and analyze a variety of shapes within a given category. The key takeaway is that shapes are represented as sets of points, allowing for mathematical comparisons and analysis of variations.
The field of SSM has roots reaching back to the late 1980s and truly blossomed in the 1990s. A crucial moment was the development of Active Shape Models (ASMs) by Cootes and colleagues, with their first influential paper published in 1987. You can delve deeper into the history of SSM here.
From Research to Reality: SSM's Impact on Patient Care
SSM isn't just a theoretical concept; it's actively changing how doctors diagnose and treat patients. Imagine a surgeon preparing for a complex spinal reconstruction. Using SSM, they can create a patient-specific spinal model to design a perfectly fitted implant. This is precision medicine at its finest.
SSM is also proving essential for early disease detection. By analyzing subtle shape changes in organs like the brain or lungs, doctors can spot early warning signs of conditions like Alzheimer's or lung cancer, often before traditional methods can detect them. These early diagnoses enable timely interventions, significantly improving patient outcomes.
The Future of SSM: Unlocking the Potential of Precision Medicine
The future of SSM is deeply connected to the advancement of personalized medicine. As we gain a more profound understanding of individual anatomical variation, the possibilities for tailored treatments expand.
Imagine using SSM to predict how a patient's heart will respond to a specific medication. This allows doctors to optimize drug effectiveness and minimize side effects. That’s the exciting potential of SSM – a future where medical care is truly personalized, precise, and predictive.
Making Sense of PCA: The Math Behind the Magic
Principal Component Analysis (PCA) sounds complex, but imagine it as a skilled artist sketching a portrait. Instead of painstakingly drawing every freckle and wrinkle, the artist focuses on the key features—the curve of a jawline, the spacing of the eyes, the shape of the nose—that truly capture the subject's likeness. PCA does much the same with data. It sifts through complex datasets and identifies the most important features, the principal components, that best describe the variation within the data.
This is incredibly useful in statistical shape modeling, particularly in medical imaging. Imagine trying to analyze thousands of 3D models of hearts, each with millions of data points. PCA helps us cut through the noise and extract the core shape differences. It's like finding the signal within the static.
Dimensionality Reduction and Variance Capture: The Core of PCA
So how does PCA actually work? It boils down to finding the directions of greatest variance in your data. Think of squeezing a stress ball. The direction in which it bulges out the most represents the largest variance. These directions become the principal components, forming a new, simplified coordinate system. This process, called dimensionality reduction, allows us to represent complex data with fewer variables while retaining the most important information.
To visualize this, let's look at a simple 2D example from the scikit-learn documentation:
This image shows how PCA finds the axes of greatest variance (the principal components) and uses them to represent the data in a new, lower-dimensional space. This simplification makes the data much easier to analyze and interpret.
Now, the beauty of PCA is that it doesn't just find these components; it ranks them by importance. The first component captures the most variance, the second captures the next most, and so on. This is crucial because it allows us to prioritize the most influential factors.
For instance, a study on cervical spine morphology used PCA to analyze 3D models from five individuals, each represented by up to 54,960 nodes. Astonishingly, just the first few principal components captured over 95% of the shape variations! You can delve into the specifics of this study here.
Why PCA is Essential for Statistical Shape Modeling
PCA isn't just a data simplification tool; it's a powerful lens for understanding anatomical variation. By identifying principal components, researchers can:
-
Quantify shape differences: Instead of relying on subjective observations, PCA provides objective numerical measures. It's like having a precise ruler for measuring shape.
-
Build statistical models: The principal components become the foundation for building models that can predict shape changes related to factors like age, disease, or genetics.
-
Visualize shape variation: PCA allows us to visualize how shapes change along the principal components, offering intuitive insights into complex anatomical variations. It's like creating a movie of how shapes morph and evolve.
Let's take a look at how PCA performs across different anatomical structures:
PCA Performance in Different Anatomical Structures
Comparison of how PCA captures shape variation across different body parts and the number of components needed for accurate representation
| Anatomical Structure | Variance Captured (%) | Components Needed | Typical Dataset Size |
|---|---|---|---|
| Hand | 90 | 5 | 100-200 |
| Heart | 95 | 10 | 50-100 |
| Brain | 98 | 20 | 20-50 |
| Face | 92 | 8 | 200-500 |
This table showcases how PCA's efficiency can vary depending on the complexity of the anatomical structure. Notice how fewer components are needed for simpler structures like the hand compared to more intricate ones like the brain.
The table highlights the significant variance captured by PCA with relatively few components, emphasizing its efficiency in representing shape information. This power of PCA makes it a cornerstone of statistical shape modeling, allowing for a more profound understanding of shape variation in relation to function and disease. This in turn paves the way for improved diagnostics and personalized treatments.
Active Models That Actually Work: ASM and AAM in Action
Building upon the foundation of Principal Component Analysis (PCA), we have Active Shape Models (ASMs) and Active Appearance Models (AAMs). These models bring a dynamic element to statistical shape modeling. Think of it like this: imagine combining the keen eye of an experienced doctor, trained to spot subtle anatomical differences, with the unwavering precision of a mathematical algorithm. That’s the power of ASMs and AAMs. They don't just passively analyze; they actively learn and adapt.
ASMs and AAMs are intelligent pattern recognition systems, trained to “see” anatomical structures much like a medical expert would. They go beyond static analysis by actively fitting themselves to new medical images, predicting the location of important anatomical landmarks. This active fitting process is key because it allows the models to handle the natural variations we see in real-world medical data.
This image from Wikipedia illustrates how an ASM iteratively fits to an image. Notice how the initial placement of the model is refined step by step, gradually aligning with the target shape. The model actively deforms to match the image’s features—a perfect example of the dynamic nature of ASMs.
Active Shape Models: Focusing on the Contours
Imagine a flexible wireframe, like a sculptor’s initial sketch, representing an anatomical structure – let's say, the outline of a hand. This wireframe is constructed from a series of interconnected landmark points. PCA defines how these points can move and deform, capturing the typical variations you might see in different hand shapes.
Now, when presented with a new image of a hand, the ASM actively searches for the best fit. It adjusts its shape, like bending the wireframe, to match the contours of the hand in the image. It's similar to adjusting a template until it perfectly overlays the target object.
Active Appearance Models: Incorporating Texture and Intensity
AAMs build on the ASM concept, adding another layer of sophistication. Instead of just the wireframe of the hand, imagine also capturing the skin tone, wrinkles, and other visual details. AAMs incorporate both shape and appearance information. They consider not only the contours but also the texture and intensity patterns within the shape itself.
This added dimension makes AAMs especially powerful when the appearance of tissues—variations in density or color, for example—provides essential diagnostic information. Imagine analyzing medical scans: an AAM could differentiate between healthy and diseased tissue based not only on the organ's shape but also on subtle differences in tissue appearance. This leads to a more nuanced and accurate analysis. Understanding data distribution is crucial; tools like a covariance matrix calculator can be incredibly helpful for this.
Comparing ASM and AAM
To understand the key differences between these two models, let's look at a comparison table:
To help clarify the distinctions between ASMs and AAMs, the following table summarizes their key features, advantages, and typical applications:
| Feature | Active Shape Models | Active Appearance Models | Best Use Cases |
|---|---|---|---|
| Input Data | Primarily shape (landmarks) | Shape and appearance (texture, intensity) | |
| Complexity | Less complex | More complex | |
| Computational Cost | Lower | Higher | |
| Accuracy | High for well-defined shapes | Potentially higher when appearance is informative | |
| Examples | Identifying bones in X-rays | Analyzing tissue characteristics in MRI scans |
This table highlights how ASMs, focusing primarily on shape, are less computationally intensive and excel with clearly defined structures. AAMs, incorporating appearance data, offer higher potential accuracy when texture and intensity variations are diagnostically significant, but at a higher computational cost.
ASM and AAM in Clinical Practice
The ability to combine shape and appearance information, particularly with AAMs, has proven incredibly useful in various medical applications. In cardiac imaging, for instance, AAMs can track changes in heart wall thickness and motion over time, providing crucial information about heart function. In oncology, AAMs can help differentiate between benign and malignant tumors based on both their shape and appearance, potentially leading to earlier and more accurate diagnoses. These active models are not only speeding up medical diagnosis but also making it more precise, ultimately contributing to more effective and personalized patient care.
Real Clinical Impact: Where Lives Are Changed
Statistical shape modeling (SSM) is moving beyond research labs and actively changing how healthcare is delivered. Imagine a cardiologist predicting heart failure months before symptoms appear, or an orthopedic surgeon creating a hip implant perfectly matched to a patient's anatomy. These aren't futuristic scenarios, they are happening in hospitals and clinics right now.
This progress is fueled by SSM’s ability to identify tiny anatomical differences, often invisible to the naked eye. Consider the difficulty radiologists face when searching medical images for the earliest hints of disease. These subtle changes, barely noticeable even to trained professionals, can be the difference between early, effective treatment and a missed diagnosis. Statistical shape modeling empowers radiologists to spot these critical variations, improving diagnostic accuracy and leading to timelier treatment.
This screenshot from 3D Slicer, an open-source platform for medical image analysis, shows statistical shape modeling in action. The software lets clinicians and researchers interact with 3D anatomical models, making complex shape analysis accessible and intuitive. This user-friendly approach directly translates to improved diagnoses and better patient care.
Practical Applications Across Medical Specialties
The benefits of statistical shape modeling reach across a wide spectrum of medical specialties. In pediatrics, it allows for precise tracking of growth disorders, offering key insights into developmental patterns and enabling early intervention. Surgical teams use these models to plan complex procedures with greater confidence, visualizing a patient's intricate anatomy and simulating surgical outcomes.
This level of precision also extends to designing patient-specific implants and prosthetics, ensuring a perfect fit and optimal function. Researchers are also investigating how SSM can predict treatment effectiveness, paving the way for truly personalized medicine where treatment plans are tailored to individual patient characteristics. This ability to personalize treatments based on shape analysis represents a significant step towards more effective and efficient healthcare.
From Lab to Bedside: Real-World Challenges and Successes
Implementing statistical shape modeling in real-world clinical settings isn't always easy. It requires addressing practical challenges, from data acquisition and processing to integration with existing clinical workflows. However, the successes are compelling. SSMs have been used to analyze multi-organ anatomies with shared boundaries, helping to detect subtle morphological biomarkers for disease. These models have even demonstrated the ability to predict osteoarthritis risk with up to 85% accuracy in recent multi-center trials. Discover more insights.
The Future of Personalized Medicine
The real clinical impact of statistical shape modeling extends beyond current applications. It’s about the potential to fundamentally change how we approach healthcare. Think of a future where diseases are diagnosed earlier, treatments are more effective and personalized, and surgical procedures are planned with incredible precision. These are the possibilities that statistical shape modeling is unlocking, moving us closer to healthcare truly tailored to each individual. These aren’t just small improvements; they represent a fundamental shift toward predictive, preventative, and personalized care. Every hour invested in refining these mathematical models brings us closer to a future of better health for everyone.
Building Models That Work: Your Implementation Roadmap
Creating a statistical shape model isn't a simple task. It's more like crafting a precision instrument, where every step, every decision, plays a crucial role in the final outcome. We're talking about a journey that starts with raw medical scans and culminates in reliable clinical insights. It's a complex pipeline, and we'll walk through it together, exploring the technical hurdles and practical choices along the way.
Data Acquisition and Preprocessing: Laying the Foundation
Think of building a house. A shaky foundation spells disaster, right? Similarly, in statistical shape modeling, the quality of your initial data is paramount. If you start with flawed data, you'll inevitably end up with an unreliable model. This first stage involves gathering a diverse and representative collection of medical images directly related to your research question.
This infographic illustrates some common challenges in this process:
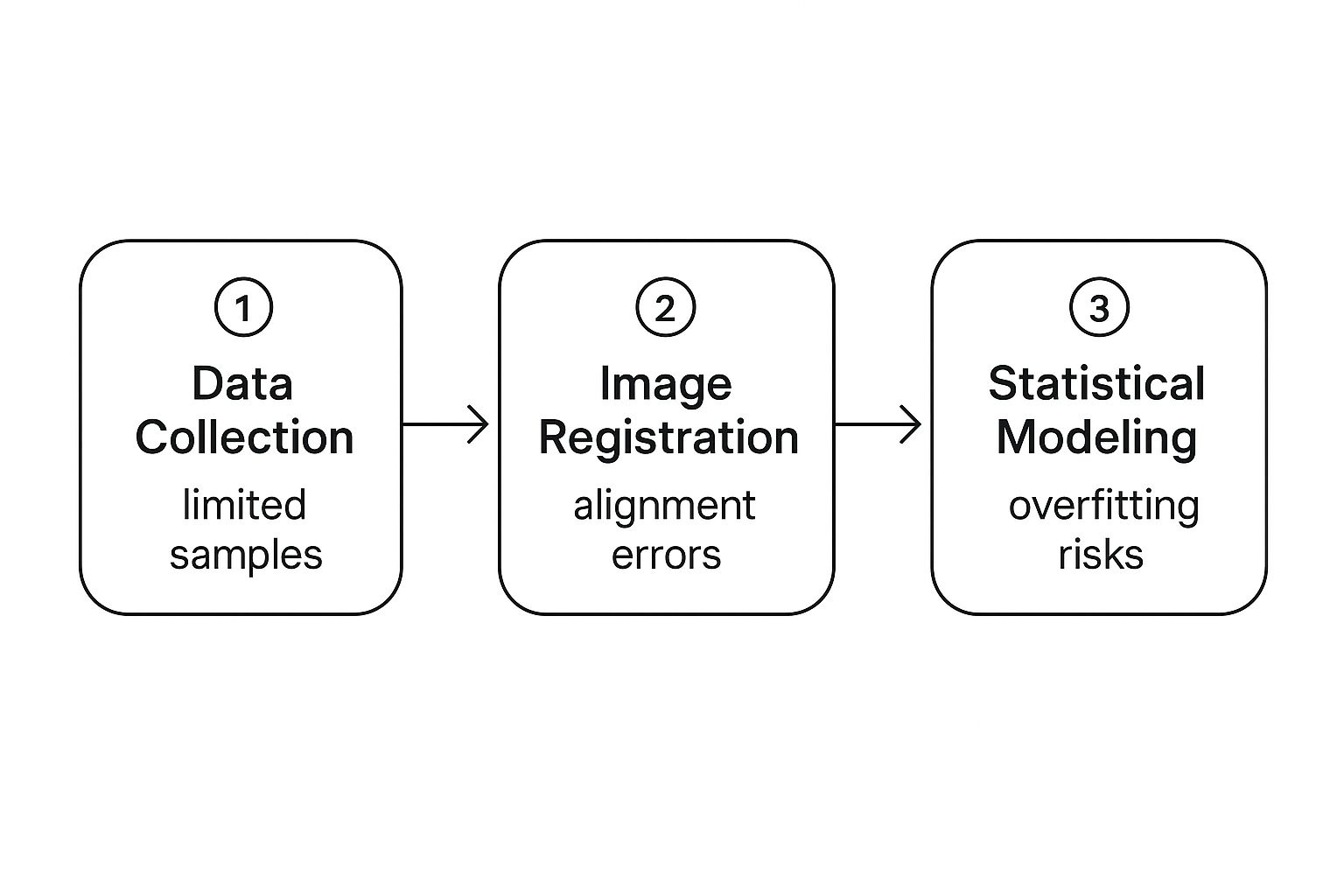
Notice how problems in one stage can cascade into the next. For example, if your data collection is inadequate, it might lead to alignment issues during image registration. This, in turn, can increase the likelihood of overfitting – a situation where your model performs well on the data it was trained on but poorly on new, unseen data – during the statistical modeling phase.
Mesh Generation and Landmark Placement: Defining the Shape
Once you have a solid dataset, the next step is to create a digital representation of the anatomical structures you’re interested in. This involves generating a mesh – imagine a wireframe model of a sculpture. It's a network of connected points that define the surface of the structure.
Then comes landmark placement. These landmarks are specific points on the mesh that correspond across all your samples. They're like anchors, ensuring that the same point on each mesh represents the same anatomical location – this is called correspondence. Accurate landmark placement is crucial for a valid statistical model. There are various techniques for mesh generation and landmark placement, and selecting the best one depends on the specific anatomy and your research question.

This screenshot is from ShapeWorks, an open-source platform for statistical shape modeling. It’s a great example of how software can simplify the complex process of mesh generation and landmark placement.
Statistical Modeling and Validation: Building and Testing the Model
Now, with preprocessed data and established correspondences, you can build your statistical shape model. This often involves using Principal Component Analysis (PCA) to identify the main ways shapes vary in your dataset. Think of these principal components as the key characteristics that distinguish one shape from another.
Validation is essential to confirm that your model is accurate and reliable. A common method is to split your data into training and testing sets. You use the training set to build the model and then test its performance on the testing set. This helps prevent overfitting—remember, that’s when your model is like a student who memorizes answers instead of understanding the material.
Tools and Best Practices: Ensuring Success
Choosing the right tools and sticking to best practices is vital. There are numerous options for statistical shape modeling, from open-source platforms like ShapeWorks to commercial software. The best choice depends on your specific needs and resources.
Successfully implementing statistical shape modeling demands careful consideration of every stage, from data collection to model validation. A methodical approach and attention to detail are essential. By utilizing the right tools and following best practices, researchers can transform raw medical data into valuable analytical tools for clinical applications, ultimately leading to improved patient care. Rigorous quality control at each step ensures the final model accurately captures shape variations, allowing for reliable predictions and impactful clinical use.
The Art of Shape: Where AI Meets Statistical Shape Modeling
Statistical shape modeling (SSM) has been a cornerstone of medical image analysis for years. Think of it as creating a blueprint, an average shape, from a collection of similar objects – like charting the "average" human heart from scans of thousands of patients. But traditional SSM sometimes struggles with the sheer complexity and nuance of real-world anatomy. Imagine trying to average the shapes of clouds – their variability makes it a tricky task.
That's where Artificial Intelligence (AI) steps in, bringing its power to bear on every part of the SSM process, opening up possibilities we could only dream of before.
Landmark Automation: AI's Precise Assistant
Consider the painstaking task of landmark placement. This involves manually marking corresponding points on hundreds, even thousands, of 3D models. It's not only tedious but also prone to human error. Imagine trying to pinpoint the exact same spot on a hundred slightly different leaves.
Now, deep learning algorithms can automate this process. They act like a tirelessly precise assistant, speeding things up dramatically and ensuring consistency. This frees up researchers to focus on what the landmarks mean rather than the tedious work of placing them.
Unveiling Hidden Patterns: Deep Learning's Insight
AI is also transforming how we extract information from shape data. Traditional methods often rely on pre-defined features, like measuring the length or width of a structure. This limits our ability to spot complex, non-linear relationships. It’s like trying to understand a symphony by only listening to individual notes.
Deep learning networks, on the other hand, learn intricate patterns automatically. They can uncover subtle connections between shape and disease that might be missed by the human eye, moving us from manual feature engineering to automated discovery. It’s like listening to the whole symphony at once, allowing us to appreciate the complex interplay of instruments and melodies.
The screenshot above, from the PyTorch Vision library, showcases some of the powerful deep learning tools available for computer vision tasks. These open-source libraries offer pre-trained models and efficient functions for tasks like image segmentation and object detection, making advanced AI techniques accessible to researchers working with medical images. This readily available technology accelerates the development of more accurate and robust shape modeling techniques.
For the mathematical side of SSM, especially implementation, a Statistics AI Solver could be a useful resource.
Scaling Up Research: Handling Massive Datasets
One of the biggest advantages of integrating AI into SSM is its ability to handle huge amounts of data. In large-scale studies, especially those involving international patient registries, SSMs have been used to analyze populations ranging from 1,000 to 10,000 individuals across Europe, North America, and Asia, with the top three to five principal components usually capturing over 90% of the shape variations. Discover more insights. Analyzing data on this scale with traditional SSM techniques would be incredibly challenging, if not impossible. AI allows us to extract meaningful insights from these large, complex datasets, accelerating the pace of medical discovery. This enables researchers to pinpoint small but important shape differences across diverse populations, opening up new avenues for more personalized medicine.
Real-Time Applications: Transforming Clinical Practice
AI also paves the way for real-time clinical applications of SSM. Imagine a surgeon instantly comparing a patient's anatomy to a statistical model during an operation. Or a diagnostic tool that analyzes a medical image in seconds, providing immediate feedback to the doctor. These applications are becoming a reality thanks to advancements in AI and computing power. This real-time potential holds tremendous promise for improving surgical precision, accelerating diagnoses, and ultimately, improving patient care. This speed and efficiency could revolutionize time-sensitive medical scenarios.
The Path Forward: Navigating Challenges and Embracing Opportunities
While the potential of AI in SSM is vast, challenges remain. Ensuring the reliability and interpretability of deep learning models is crucial, especially in medical settings where accuracy and trust are paramount. We also need to address data bias and ensure equitable access to these technologies. Despite these challenges, the future of SSM is clearly linked to the continued development of AI. This powerful combination is reshaping medical image analysis, leading to more accurate diagnoses, more personalized treatments, and a healthier future for all. The possibilities are only beginning to emerge.
Your Path to Statistical Shape Modeling Success
Success in statistical shape modeling (SSM) isn't just about mastering the technical details; it's about understanding the nuances of working with real-world medical data. Think of it like a seasoned chef versus someone who just follows a recipe. Both can technically cook, but the chef's experience leads to a far superior dish. Similarly, experienced researchers in SSM have a knack for navigating the complexities of medical data, and that's what separates impactful projects from purely academic exercises. This section shares insights from those experts, guiding you on everything from selecting appropriate sample sizes to validating your results in clinically meaningful ways.
Navigating the Landscape: From Data to Insights
SSM provides a powerful set of tools for analyzing how anatomical structures vary. Imagine it like a craftsman's toolbox – full of potential, but only effective in skilled hands. Building a successful SSM pipeline means understanding each step intimately, from collecting the initial data to verifying the final model. It's not simply about running software; it's about making informed decisions at every turn.
This screenshot, from the ShapeWorks GitHub page, exemplifies the importance of community in this field. ShapeWorks is an open-source platform for SSM, and the open-source nature encourages researchers to share code, contribute to development, and collectively improve the available tools. This collaborative environment fosters innovation and leads to more robust methods for shape analysis.
Emerging Trends and Ethical Considerations
Machine learning is transforming how we analyze and interpret shape. Much like how a microscope opened up a new world of microscopic life, machine learning is revealing intricate patterns and relationships in shape data that traditional methods might miss. These models are not just automating tasks; they're unlocking deeper insights, propelling new discoveries, and accelerating progress in the field. For a deeper look into how Machine Learning is influencing other sectors, check out this article on Machine Learning in Education.
However, these powerful tools come with important ethical considerations. Analyzing human anatomical data carries responsibilities related to patient privacy and data security. Think of it like a doctor's oath – we must ensure that data is anonymized, protected, and that all research is conducted ethically and responsibly. Furthermore, the regulatory landscape surrounding clinical applications is constantly evolving, and staying abreast of these changes is crucial for anyone in this field.
Building Expertise: Interdisciplinary Skills
SSM sits at the crossroads of mathematics, medicine, and computer science. Excelling in this area requires a blend of skills, much like a multi-instrumentalist in a band. A strong foundation in statistical concepts is essential, alongside a familiarity with medical imaging techniques and anatomical structures. Programming skills are also increasingly important, especially with the growing role of AI and machine learning in shape analysis.
Developing expertise in SSM is a journey that combines theoretical knowledge with hands-on experience. Engaging with the research community, attending conferences, and contributing to open-source projects are all valuable ways to learn and keep up with advancements. This continuous learning is key to staying at the forefront of this dynamic field.
From Research to Real-World Impact
The real power of SSM lies in its potential to improve human health. Whether you're a researcher developing algorithms or a clinician using these tools, the ultimate goal is to translate research findings into tangible benefits. This requires more than just technical proficiency; it demands an understanding of the clinical context, a commitment to ethical practices, and a genuine focus on patient well-being. By embracing these principles, we can ensure that SSM continues to drive progress and transform healthcare.
Interested in applying AI to medical imaging? Explore how PYCAD can help you optimize medical devices and enhance patient care. Learn more about the possibilities and see how PYCAD can contribute to better healthcare outcomes.






