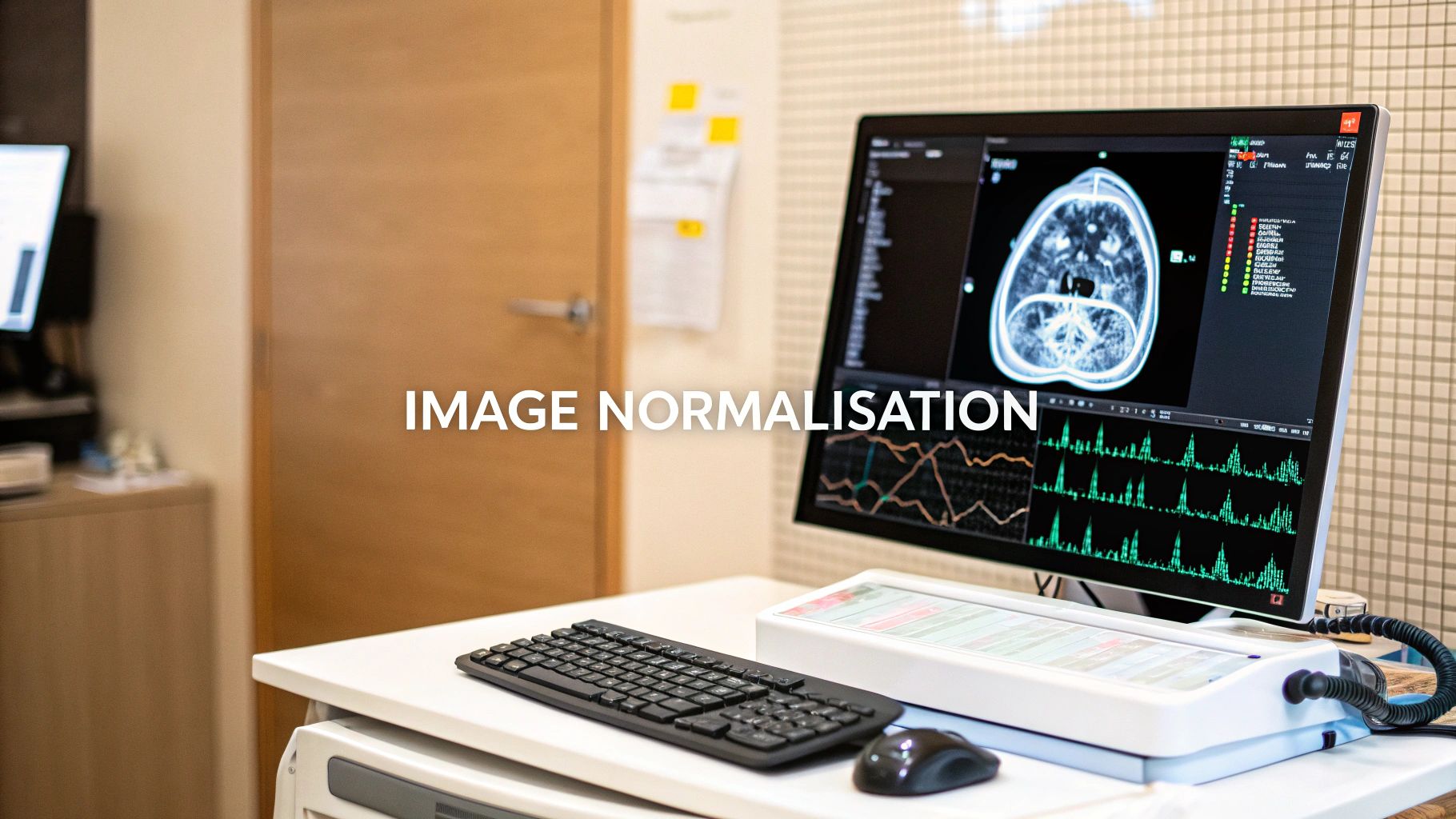
Image normalization is a fundamental step in data preprocessing where we adjust the range of pixel intensity values to a standard, common scale. Think of it as creating a consistent "language" for all images. This ensures that variations in brightness or contrast don't confuse an AI model during training, a critical step for building reliable and accurate models, especially in a sensitive field like medical imaging.
Understanding Image Normalisation and Its Importance

Imagine you're trying to compare two photos of the same building. One was shot on a bright, sunny day, while the other was taken on a gloomy, overcast afternoon. The colors, brightness, and contrast would be completely different, making it tough to compare the building’s actual structure in a fair, apples-to-apples way.
This is precisely the challenge AI faces when analyzing images. The core information—the building itself—is the same, but its representation is skewed by external factors like lighting. Image normalisation solves this by acting as a great equalizer.
It’s a data preparation technique that transforms the pixel intensity values of an image into a standardized range. The process doesn't change the content of the image; it just adjusts how the pixel values are represented mathematically.
Why Is Standardizing Pixel Values Critical?
Without normalization, an AI model could easily mistake superficial variations for meaningful features. It might learn that brighter images signal one outcome and darker images another, completely missing the anatomical details that truly matter. In medical AI, this kind of mistake can have serious consequences.
By putting every image on a level playing field, normalisation ensures that the AI model focuses on learning genuine biological patterns rather than being misled by inconsistent data acquisition. It forces the model to look deeper, beyond the surface-level noise of varying brightness and contrast.
Essentially, normalizing image data prevents the model from learning the wrong lessons. It’s a foundational step that directly impacts the reliability, accuracy, and generalizability of any computer vision model.
Problems Solved By Image Normalisation
Normalization isn't just a "nice-to-have"; it's a powerful tool that directly tackles several common issues that plague raw image datasets, especially in complex fields like medical imaging.
The table below breaks down the specific problems that normalization addresses, showing just how essential it is for training AI models you can actually trust.
| Problem | How Normalisation Helps |
|---|---|
| Varying Illumination | Corrects for differences in lighting, ensuring an object looks the same to the AI whether it was scanned in a bright room or a dim one. |
| Inconsistent Contrast | Adjusts images with poor or extreme contrast to a standard level, making subtle features more consistently visible to the algorithm. |
| Different Sensor Calibrations | Medical scanners and cameras have unique hardware properties. Normalisation mitigates these differences, creating uniformity across data from multiple sources. |
| Slower Model Training | When pixel values are scaled to a smaller range (like 0 to 1), the machine learning algorithm can find a solution much more quickly and efficiently. |
By tackling these issues head-on, image normalization makes the entire AI development pipeline more robust. It's a non-negotiable step for any serious computer vision project, paving the way for models that are not just accurate but also trustworthy enough for real-world applications.
Why Normalization is So Critical in Medical Imaging
In the world of medical imaging, you'd think an MRI of a brain is an MRI of a brain. But that's rarely the case. A scan from one hospital can look wildly different from one taken at another facility, even when looking at the same anatomy. This isn't an error; it's just a reality of how this complex data is captured.
These variations come from all sorts of places that are tough to pin down in a busy clinical environment. Every single piece of the puzzle—from the scanner hardware to the software it runs—leaves its own fingerprint on the final image.
Where Does This Inconsistency Come From?
The variability we see isn't just random noise. It comes from specific, identifiable sources that all pile up, creating a dataset that's difficult for an AI to learn from. Once you know the sources, you can start to fix the problem.
Here are the main culprits:
- Different Scanner Hardware: A GE scanner simply doesn't "see" the world the same way a Siemens or Philips scanner does. Their images have fundamentally different characteristics.
- Varying Acquisition Protocols: Even on the exact same machine, tiny tweaks to scanning parameters—like the echo time in an MRI—can change the pixel intensities across the entire image.
- Patient-Specific Factors: People are different. A patient's body composition or even their hydration level can subtly alter the resulting scan.
- Post-Processing Software: The software that pieces the raw data into a viewable image often applies its own automatic adjustments, adding yet another layer of variability.
This creates a huge roadblock for any AI model. If you train an algorithm on this raw, messy data, it might start learning all the wrong things. For instance, it could learn that images from "Hospital A's" scanner are more likely to contain a tumor, not because of the patient's biology, but because of that specific machine's intensity signature. The model learns a misleading shortcut.
An AI diagnostic tool should be an expert in finding disease, not in identifying scanner brands. Without the normalisation of image data, the model picks up on these bogus signals, resulting in a tool that can't be trusted when it sees data from a new hospital or scanner.
Leveling the Playing Field for AI
This is precisely where image normalization steps in. It's the great equalizer. Normalization erases all those superficial, machine-specific differences and forces every image onto a common scale. It standardizes the dataset, so the AI model can finally focus on what truly matters: the anatomical structures and pathological signs within the patient's tissue.
It’s a bit like tuning an orchestra. If every instrument is tuned to a different reference pitch, all you get is chaotic noise. But when they’re all calibrated to the same standard, you get harmony. Normalization does the exact same thing for a massive collection of medical images.
For a company like PYCAD, which is dedicated to building reliable medical AI, this isn't an optional step—it's foundational. It’s how we make sure our models are not just accurate in a controlled lab setting but are also dependable and generalizable out in the real world. This careful data preparation is the bedrock of building safe and effective AI tools that clinicians can actually rely on to improve patient care.
Core Image Normalization Techniques Explained
Getting a handle on image normalization doesn't require a Ph.D. in statistics. The formulas might look a bit intimidating at first glance, but the ideas behind them are surprisingly straightforward. By breaking down the most common methods, we can build a solid foundation for understanding how they work and, more importantly, when to use them for the kind of medical imaging tasks we tackle at PYCAD.
Think of these techniques as different tools in your workshop. Each one is designed for a specific job, and the real skill lies in knowing which one to grab.
Min-Max Scaling: The Great Equalizer
The most direct approach is Min-Max Scaling. Imagine you have a box of rubber bands, all different lengths. Your goal is to make them fit perfectly into a small, uniform container. To do this, you’d stretch the short ones and shrink the long ones until they all had a consistent length.
That's exactly what Min-Max Scaling does for pixel values. It takes every pixel intensity in an image and mathematically "stretches" or "squishes" it to fit neatly within a specific range, usually 0 to 1 or -1 to 1.
This method is popular for a few good reasons:
- Simplicity: It’s incredibly easy to understand and implement, making it a perfect starting point.
- Guaranteed Range: It ensures all pixel values fall within a predictable, bounded range. This is a huge plus for certain neural network architectures that expect inputs between 0 and 1.
- Preserves Ratios: It keeps the relative relationships between pixels intact. If one pixel was twice as bright as another before scaling, it remains proportionally so afterward.
But its greatest strength—simplicity—is also its biggest liability. Min-Max Scaling is very sensitive to outliers. A single, unusually bright or dark pixel, maybe from a scanner artifact, can completely throw off the scaling for the entire image. That one outlier becomes the new "max" or "min," forcing all the other legitimate pixel values into a much narrower band and potentially hiding the very details you want the AI to see.
Z-Score Normalization: Finding the Center
A much more robust and widely used method is Z-score Normalization, often called standardization. Instead of cramming values into a fixed range, this technique recalibrates the data around its own center. It’s like a teacher grading on a curve. If the class average on a tough exam was a 50, the teacher might adjust all the scores so the new average is a 75. Z-score does something similar, but it always aims for an average (mean) of zero.
It transforms the pixel data so that it has a mean of 0 and a standard deviation of 1. This process effectively reframes each pixel's value in terms of how far it deviates from the image's average intensity.
The real magic of Z-score normalization is how it handles outliers. By standardizing the distribution, it helps those unusual values—subtle but potentially critical anomalies in a scan—stand out, making them much easier for an AI model to spot.
This is one of the most trusted techniques in data science. Statistical experiments have shown that datasets normalized with the Z-score method can lead to significant bumps in model accuracy, sometimes by as much as 15%. This is especially true when the original pixel intensities follow a somewhat bell-shaped curve. You can dive deeper into these statistical approaches and their effects by exploring key normalization methods in statistical analysis.
Here’s a quick side-by-side look at how these two methods stack up.
| Feature | Min-Max Scaling | Z-Score Normalization |
|---|---|---|
| Primary Goal | Scale values to a fixed range (e.g., 0 to 1). | Center data with a mean of 0 and standard deviation of 1. |
| Output Range | Bounded (e.g., [0, 1]). | Unbounded (values can be > 1 or < -1). |
| Sensitivity to Outliers | High. One extreme value can skew all others. | Low. More robust against the influence of outliers. |
| Best Use Case | When your model requires a strict input range. | When your data is roughly bell-shaped or contains outliers. |
Ultimately, the choice between them comes down to your data and your model. While Min-Max is simple and effective for some tasks, Z-score's stability often makes it the superior choice for the complex and varied data we encounter in medical imaging.
Comparing Popular Image Normalisation Methods
Picking the right image normalisation technique isn't a one-size-fits-all deal. It's a strategic choice that hinges entirely on your specific dataset and what your model needs to succeed. The perfect method for one project might completely derail another. The best way to get a feel for this is to put them head-to-head and understand the trade-offs.
Let's start with a visual to see how these methods take raw pixel data and whip it into shape.
The infographic below shows how different techniques transform raw pixel values, remapping them to new, standardized scales. Think of it as translating different dialects into one common language the model can understand.
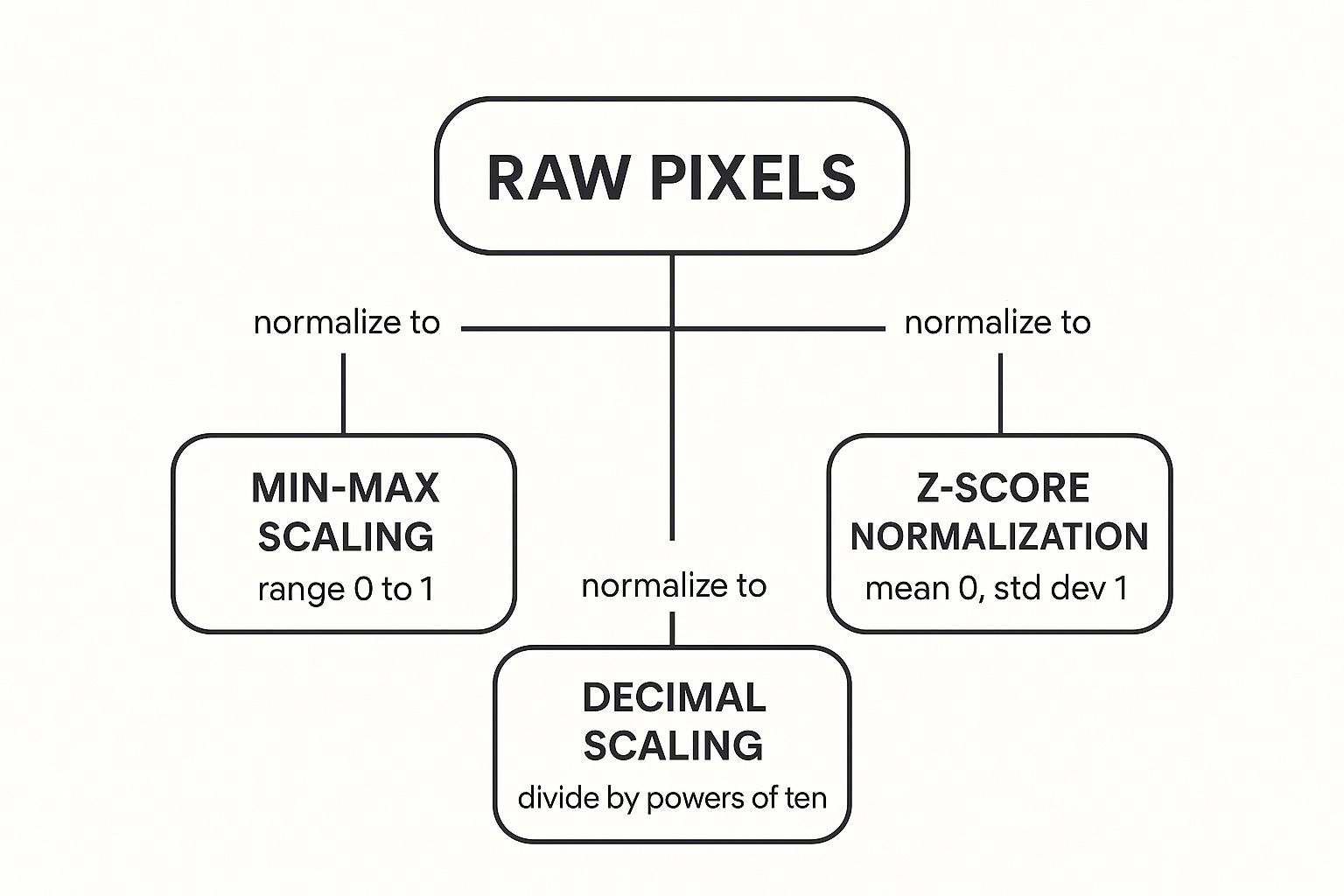
As you can see, the end goal varies—some methods lock the data into a neat 0 to 1 range, while others re-center everything around a mean of zero. Now, let’s dig into what this means in practice.
General-Purpose Normalisation Techniques
For most applications, the choice usually comes down to a few trusted workhorses. Min-Max Scaling and Z-score Normalisation are the heavy lifters of data preprocessing, but they have very different personalities. A third, Histogram Equalization, takes a different path entirely by focusing on contrast.
To make the choice clearer, this table breaks down the pros, cons, and best-use cases for these common methods.
Comparison of Image Normalisation Techniques
A detailed look at the advantages, disadvantages, and best-use cases for common image normalisation methods.
| Technique | Primary Advantage | Key Disadvantage | Best Used For |
|---|---|---|---|
| Min-Max Scaling | Guarantees all pixel values fall within a fixed range, typically 0 to 1. | Extremely sensitive to outliers; a single unusually bright or dark pixel can skew the entire image's scaling. | Models that require inputs within a strict, bounded range. Simple datasets without significant outliers. |
| Z-Score Normalisation | Highly robust against outliers. Centers the data on a mean of 0, which can speed up model training. | The output is not bounded to a specific range, which can be an issue for some models. Assumes a somewhat bell-shaped data distribution. | Datasets with known outliers or when the distribution of pixel intensities is roughly normal. A strong default choice for medical imaging. |
| Histogram Equalization | Maximizes image contrast by spreading out the most frequent pixel intensity values. | Can produce unnatural-looking images and may amplify background noise, treating it as significant information. | Enhancing contrast in images where details are washed out, though less common as a primary normalisation method for AI model training. |
Understanding these differences is crucial. The simplicity of Min-Max Scaling is tempting, but its vulnerability to outliers makes it a risky bet for medical scans. Imagine an imaging artifact skewing the scale—it could compress the intensity range of actual tissue, hiding critical diagnostic details.
Z-score, on the other hand, handles those unexpected values with much more grace. It's often the go-to method for complex datasets like those handled by PYCAD, as it preserves the importance of unusual data points without letting them hijack the entire scale.
Specialized Normalisation for Medical Imaging
While the general methods are powerful, some challenges in medical imaging call for more specialized tools. This is especially true in neuroimaging, where precision is everything and standard techniques can sometimes miss the mark.
A great example of this is White-Stripe Normalisation. This technique was developed specifically for analyzing brain MRIs, particularly for conditions like multiple sclerosis where lesions can throw off standard calculations.
The core idea behind White-Stripe is to normalize an image based on the intensity of healthy-looking white matter. It finds a "stripe" of normal white matter tissue and uses its intensity profile as the reference point to standardize the rest of the image.
This approach brings a game-changing benefit:
- Biological Reference: It normalizes based on a consistent biological landmark, not just the image's overall statistics. This makes it far less likely to be skewed by large lesions or other abnormalities that would wreck a Z-score calculation.
White-Stripe is a perfect illustration of how the field evolves. When standard methods aren't quite enough for a high-stakes job, researchers develop new, refined tools to meet the challenge, ensuring AI models get the most consistent and meaningful data possible.
Where Automated Image Normalisation is Headed
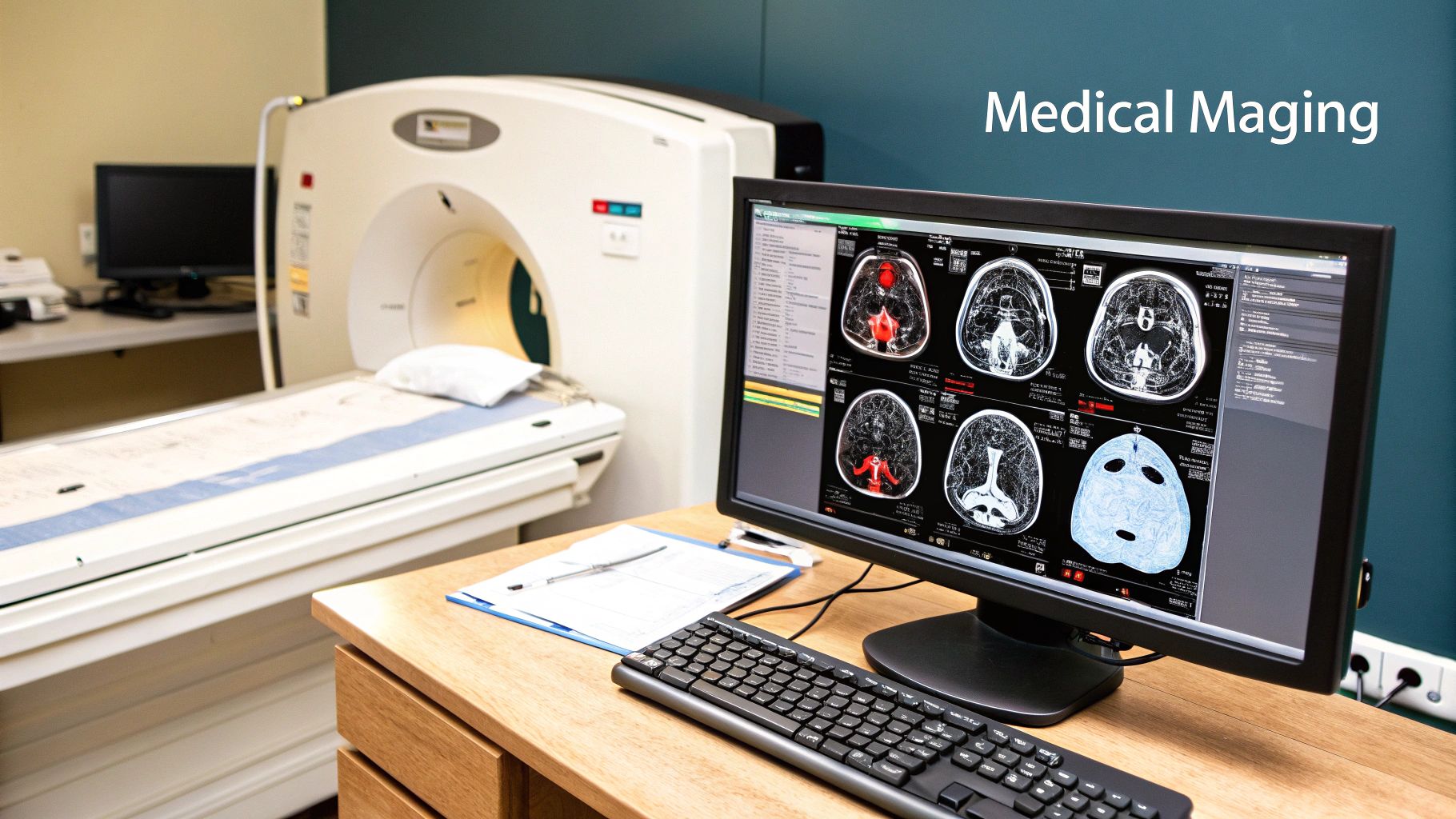
Let's be honest: manually choosing the right normalisation method for a huge, mixed bag of images is a major roadblock. It's a job that demands a lot of expertise, trial-and-error, and, most importantly, time. But what if you could just skip that whole process? That’s exactly where AI in medical imaging is going next.
The future is all about smart systems that handle the normalisation of image data automatically. We're seeing deep learning models that are trained not just to understand images, but to first get them ready for analysis.
Picture this: you have a massive, messy collection of medical scans from different hospitals, clinics, and machines. An intelligent pipeline could ingest this data, instantly figure out all the inconsistencies, and apply the perfect normalisation strategy for that specific dataset—all on its own.
A Huge Leap in Efficiency
This move toward automation isn't just about convenience; it's a complete game-changer for workflow efficiency. The whole data prep and standardization phase, which can easily eat up days of a data scientist's time, could soon be crunched down to hours or even minutes.
This kind of speed-up has massive implications for medical research and development. It frees up specialists to concentrate on what really matters—like designing better model architectures or validating clinical results—instead of being stuck in the weeds of repetitive data cleaning.
A great example from outside medical imaging really drives this home. One project involved manually standardizing 57 different survey datasets, each with its own quirks. This task used to take over 25 hours of painstaking work. With an AI-driven approach, the entire job was finished in less than an hour. That’s a mind-boggling 96% reduction in processing time and shows just how much AI can scale up these operations.
How Does Automated Normalisation Actually Work?
This next wave of automation is built on a concept called meta-learning, which is essentially "learning how to learn." Instead of just learning from the images, these models learn from the data preparation process itself. The system looks at the characteristics of a new dataset and predicts which normalisation technique will work best.
Here’s a breakdown of the automated steps:
- Dataset Characterization: First, the model analyzes the statistical profile of the images—things like pixel intensity distribution, contrast, and any unusual outliers.
- Method Selection: Based on that analysis, it picks the ideal normalisation algorithm, whether that's Z-score, Min-Max, or something more specialized for the task.
- Parameter Tuning: The system then automatically figures out the best parameters for that chosen method, making sure it’s a perfect fit for the data.
- Quality Check: Finally, it runs a quick verification to make sure the normalisation worked as expected and didn't introduce any new problems.
This intelligent, hands-off approach makes building robust, large-scale medical AI systems far more efficient and significantly less prone to human error. It democratizes access to high-quality data preparation, a cornerstone of reliable AI.
This trend isn't just limited to software, either. You can see the same principles at play in advanced hardware. For instance, the sophisticated technology inside AI tracking gimbals needs perfectly consistent image data to track subjects smoothly. Automated normalisation is what allows them to perform so well in real time.
By automating this foundational step, companies like PYCAD can dramatically speed up the entire model development cycle. The end result? More accurate and reliable diagnostic tools get into the hands of the medical community much, much faster.
Answering Common Questions About Image Normalization
As you start working with data preparation, a few questions always seem to come up. Let's tackle some of the most common ones to clear up any confusion about normalizing images and give you more confidence in the process.
Do I Really Need to Normalize My Images?
I get asked this all the time, and it's a critical question. For almost any project, especially in medical imaging, the answer is a hard no—you can't skip this step. If you do, you're essentially training your AI model to focus on the wrong things, like differences in scanner brightness instead of the actual biological patterns you need it to see.
Imagine a model trained on images from one hospital's scanner. Without normalization, it will likely fail when it sees images from another hospital. The model won't be reliable in the real world because it can't generalize. The only time you might get away with it is in a perfectly controlled setting where every single image comes from the same machine with the exact same settings—and that almost never happens.
Will Normalization Erase Important Details in the Image?
This is a common worry, but it's a misconception. Normalization doesn't erase critical information; it actually does the opposite. It highlights the important details for the AI. A good analogy is adjusting the contrast on your TV—you aren't changing the show's content, you're just making the picture clearer.
The process doesn't alter the underlying anatomy in an image. It simply recalibrates the pixel values so they're on a consistent scale. This standardization helps the model zero in on the features that matter, rather than getting distracted by noise from different imaging protocols.
So, Which Normalization Method Is the Best One?
There's no single "best" method that works for every project. The right choice really comes down to your specific data and what you're trying to achieve. That said, here are some solid guidelines:
- Z-Score Normalization: This is my go-to recommendation and a fantastic starting point for most medical imaging tasks. It’s robust, handles outliers gracefully, and centers the data, which typically helps models train faster and more effectively.
- Min-Max Scaling: You'll want to use this method when your neural network's architecture demands input values within a strict range, like 0 to 1. Just be careful—it’s extremely sensitive to outliers, which can skew your entire dataset.
- Specialized Methods: For highly specific problems, like analyzing brain MRIs with lesions, you might need a more tailored approach like White-Stripe normalization to get the accuracy you need.
Ultimately, the best technique is the one that sets your dataset up for success with your chosen model. Always take a close look at your data's characteristics before you decide.
At PYCAD, we specialize in turning these complex data prep challenges into straightforward, effective solutions. Our team has the experience to ensure your medical imaging data is perfectly prepared for building accurate and reliable AI models. See how we can help get your project off the ground by visiting us at https://pycad.co.