Understanding Quality Control in Radiology – What Really Matters
Quality control in radiology is much more than a simple series of checkboxes; it is the fundamental safety net protecting every patient. Think of it as the detailed pre-flight inspection a pilot performs before every single takeoff. It’s systematic, non-negotiable, and absolutely essential for a safe journey. In medical imaging, this "journey" is the diagnostic process, and the stakes are just as high. A strong quality control in radiology program is the invisible backbone of a facility, working to find and fix potential issues before they can ever impact a patient’s diagnosis or care.
This systematic approach isn't a new idea. The formal effort to establish quality control in diagnostic radiology grew significantly in the late 20th century. A pivotal moment came in 1977 when the American Association of Physicists in Medicine (AAPM) published a key protocol to help radiologic technologists set up quality assurance programs. Since then, QC practices have continued to develop, adapting to major technological changes like digital radiography, fluoroscopy, and advanced cardiac imaging systems. You can read more about the early AAPM quality assurance protocols and their evolution.
The Core Principles of Effective QC
At its heart, quality control is built on two core principles: consistency and accuracy. It’s about making sure that an X-ray taken on a Monday produces the same diagnostic quality as one taken on a Friday, no matter which machine or technologist is involved. It’s also about confirming that the image correctly represents the patient’s anatomy without any distortion or artifacts.
To make this happen, successful QC programs are built on several key elements:
- Acceptance Testing: This is the first, in-depth evaluation performed on new or repaired equipment before it is ever used for patient imaging. It sets the baseline for performance.
- Routine Performance Monitoring: These are the regular checks—daily, weekly, or monthly—that confirm the equipment is still operating within its set limits.
- Error Correction: When a test result falls outside the acceptable range, a clear process must be in place to find the problem, fix it, and re-test the equipment's performance.
From Compliance to Culture
While regulations establish the minimum standard, the most effective facilities go beyond just meeting requirements. They build a culture where quality is a shared responsibility. This means every team member, from the front desk staff to the lead radiologist, understands their part in maintaining high standards. For instance, a technologist who spots a slight blur on a daily test image and reports it immediately is contributing directly to patient safety.
This cultural shift turns abstract rules into practical, life-saving actions. It changes the process from a dreaded audit into a source of pride, ensuring that every CT scan, MRI, and X-ray meets the highest possible standard. It’s not just about passing inspections; it’s about a deep-seated commitment to delivering the best diagnostic information possible, which ultimately protects the health of every patient who comes through the door.
The Quality Control Team: Who Makes It All Work
Behind every clear, diagnostically useful medical image stands a dedicated team. They function like a well-orchestrated symphony to ensure precision and safety. Effective quality control in radiology is never a one-person job; it is a collaborative effort where each member plays a specific and vital role. This teamwork builds multiple layers of safety, catching potential problems long before they can affect a diagnosis.

The Architects and the Implementers
Think of the quality control team as having two main groups: the architects who design the safety systems and the implementers who execute them daily. Each person brings a unique set of skills, and all are essential for a solid QC program.
Here are the key players on this team:
- Medical Physicists: These professionals are the technical guardians of the radiology department. As the architects, they are responsible for designing and overseeing the entire QC program. Their duties include conducting yearly equipment performance evaluations, running acceptance tests on new machines, and defining the exact standards for image quality and radiation dose. They are the experts to call when complex technical issues come up.
- Radiologic Technologists: As the implementers, technologists are on the front lines of quality control. They carry out the daily and weekly QC tests, like equipment warm-up procedures and phantom image checks. Their hands-on position allows them to spot subtle, day-to-day changes in equipment performance that could signal a bigger problem down the road. Their consistent work turns the physicist's plan into a practical reality.
- Radiologists: While their main job is diagnosis, radiologists provide crucial feedback that closes the QC loop. If they see artifacts, poor image quality, or other inconsistencies, their input helps the team find and fix underlying equipment or procedural problems. Their clinical viewpoint ensures that technical standards result in images that are truly valuable for diagnosis.
- Service Engineers: These are the specialists, often from the equipment manufacturer, who handle repairs and preventative maintenance. They work in close partnership with medical physicists and technologists to troubleshoot and fix equipment malfunctions found during QC tests.
Facing Modern Challenges
This teamwork is more critical than ever, particularly with the fast pace of imaging technology development. However, creating and keeping this expert team has its difficulties. There is a recognized global shortage of clinically qualified medical physicists (CQMPs) who possess the specialized skills needed for modern radiology QC and dosimetry.
This scarcity puts pressure on facilities that are trying to keep up with digital imaging and maintain high safety standards. You can learn more about this global issue and the initiatives to address the shortage of medical physicists on IAEA.org. This human element—having the right people in the right roles—is what changes quality control from a simple checklist into a living culture of excellence and patient safety.
2. Essential QC Tests That Keep Everything Running Smoothly
Effective quality control in radiology relies on a series of carefully planned tests and checks. Think of it like a master chef who continuously tastes and adjusts a recipe throughout the cooking process. These essential protocols are the foundation of a successful QC program, making sure every piece of equipment is ready, reliable, and safe. The testing schedule is typically organized by frequency, creating a layered approach to quality assurance.
Daily, Weekly, and Monthly Checks
The core of any solid QC program is consistency. Just as you might warm up your car on a cold morning, imaging equipment needs daily procedures to confirm it's working correctly before the first patient appointment. These are often simple yet indispensable tasks.
- Daily Warm-up Procedures: These checks ensure the system is stable and ready for use. For a CT scanner, this could mean running an air calibration scan to look for artifacts. In digital radiography, it includes cleaning imaging plates and checking display monitors.
- Weekly Performance Assessments: These tests are a bit more detailed and are designed to catch problems as they emerge. A common weekly task involves scanning a phantom image—an image of a standardized object with known properties. This helps technologists spot subtle shifts in image quality, like noise or uniformity, that might go unnoticed in clinical images.
- Monthly Evaluations: These checks go deeper into specific performance metrics. This could involve verifying the accuracy of the laser lights used for positioning patients or confirming that the digital image processing software is applying corrections correctly.
Comprehensive Annual Evaluations
While daily and weekly tests monitor for immediate issues, annual evaluations confirm long-term reliability and regulatory compliance. A qualified medical physicist typically performs these, conducting a thorough assessment of the entire imaging system. Key tests include verifying beam quality and alignment.
Beam quality assessments are critical for both image quality and patient safety, confirming that the radiation output is accurate and consistent. For instance, a miscalibrated X-ray tube could deliver a higher-than-necessary radiation dose. Alignment checks are just as important. They ensure the radiation field precisely matches the area indicated by the light field, a process known as field size verification. This practice prevents unnecessary radiation exposure to parts of the body outside the imaging area. A misalignment of just 1% to 2% is often the trigger for corrective action, showing the high level of precision needed.
To give a clearer picture of how these tests fit into a broader framework, the table below outlines the essential QC checks required for different imaging modalities and their typical frequencies.
| Imaging Modality | Daily Tests | Weekly Tests | Monthly Tests | Annual Tests |
|---|---|---|---|---|
| Computed Tomography (CT) | Air/water phantom scans, display monitor check, room inspection. | Phantom image quality (noise, uniformity), CT number accuracy. | Hardcopy output quality, display monitor calibration. | Medical physicist evaluation (dose, resolution, slice thickness, laser alignment). |
| Digital Radiography (DR/CR) | System warm-up, detector calibration, cleaning of imaging plates. | Phantom image quality (uniformity, artifacts), display monitor check. | Cassette/plate inspection and cleaning, erase cycle verification. | Medical physicist evaluation (exposure index, resolution, dose, collimation). |
| Mammography | Processor QC (for film), display monitor check, phantom image acquisition. | Phantom image quality evaluation (fibers, specks, masses). | Compression check, visual checklist. | Medical physicist evaluation (dose, phantom score, AEC, collimation). |
| Magnetic Resonance Imaging (MRI) | System start-up, table positioning checks, cryogen level check. | Center frequency, signal-to-noise ratio (SNR), image uniformity using a phantom. | Geometric accuracy, slice thickness. | Medical physicist evaluation (magnetic field homogeneity, RF coil checks, image quality). |
| Table 1: Essential QC Tests by Imaging Modality | ||||
| A comparison of required quality control tests across different imaging modalities including frequency and key parameters. |
As the table shows, each modality has unique testing requirements, but the principle remains the same: regular, systematic checks ensure consistent performance. Daily and weekly tests are often handled by technologists, while more complex annual evaluations require a medical physicist.
The infographic below compares key elements of two major quality assurance standards, showing how different frameworks approach these testing protocols.
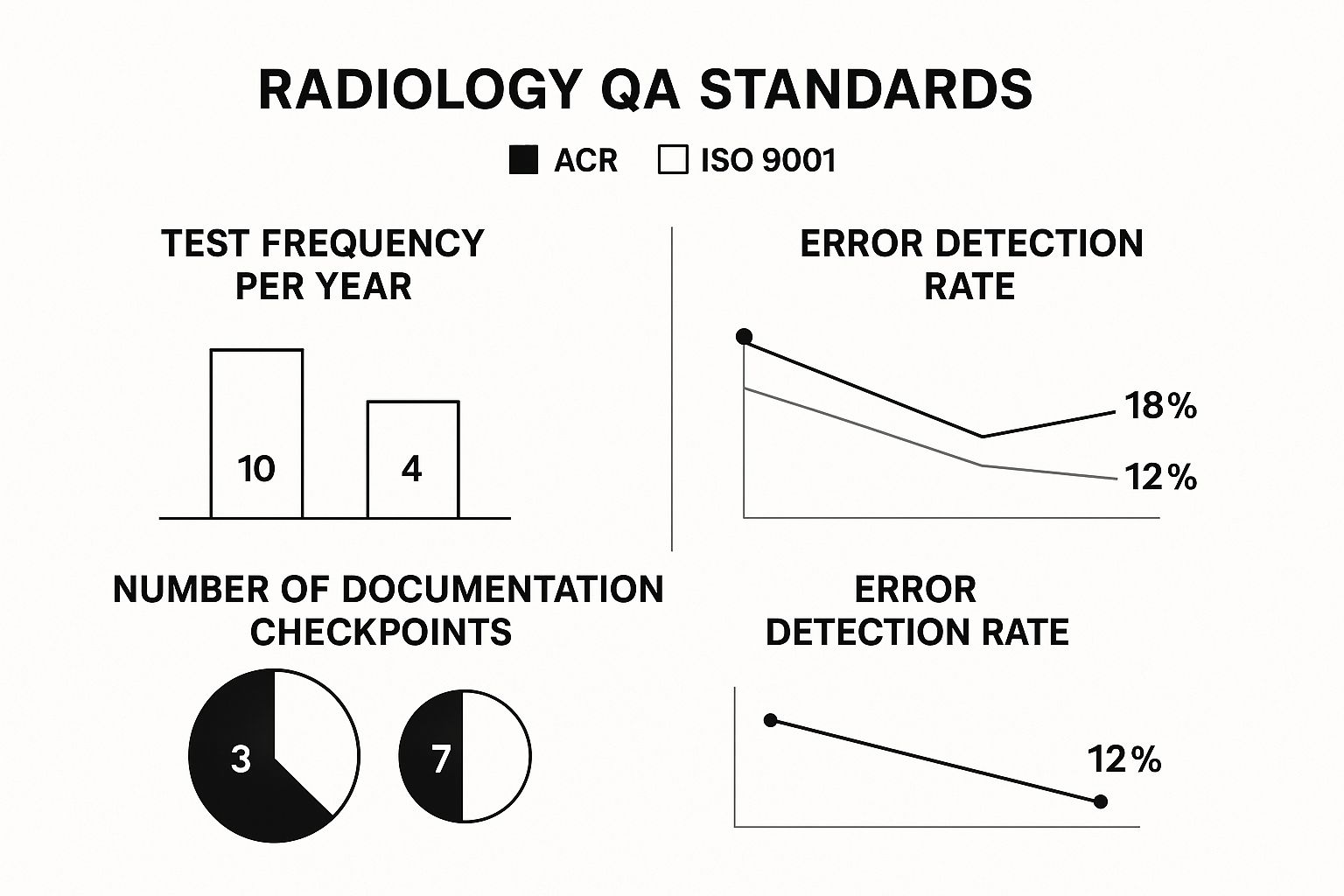
This visual shows that while standards like the American College of Radiology (ACR) are specific about test frequency, others like ISO 9001 emphasize the overall quality management system, including documentation. A complete QC program blends these scheduled tests with a strong documentation habit. Every check, from the daily warm-up log to the physicist's detailed annual report, builds a performance history for each machine. This record is essential for troubleshooting, predicting maintenance needs, and proving compliance during inspections. This organized testing ensures that a facility produces the best possible images at the lowest necessary radiation dose.
4. AI-Powered Quality Control: The Future Is Here
The world of quality control in radiology is undergoing a major technological shift. Think about how GPS transformed navigation from static paper maps to real-time, dynamic guidance. In a similar way, artificial intelligence is no longer a theoretical concept in radiology; it's actively reshaping how QC programs work. These AI-driven solutions act as a constant, vigilant partner to the QC team, creating a more proactive and precise way to ensure equipment performance and diagnostic accuracy.
From Reactive Fixes to Predictive Maintenance
Traditionally, quality control has been a reactive process. A test fails, a piece of equipment breaks down, and only then is a service engineer called to fix it. AI flips this model on its head by introducing predictive capabilities. It’s like having a smart system in your car that warns you about low tire pressure long before you end up with a flat on the side of the road. In radiology, AI algorithms can analyze performance data from imaging equipment around the clock, spotting subtle changes that are invisible to the human eye.
These platforms are designed to:
- Monitor equipment performance logs in real-time.
- Detect minor drifts in image quality metrics, such as signal-to-noise ratio or spatial resolution.
- Flag potential component failures before they happen by recognizing historical data patterns.
This allows facilities to schedule proactive maintenance during off-hours, preventing the kind of unexpected downtime that can delay patient care. For instance, an AI system might notice a tiny increase in image noise on a CT scanner over a week. It could then predict a potential X-ray tube issue and alert the team to schedule a check before it fails during a critical patient scan.
Enhancing Human Expertise, Not Replacing It
A common concern is that AI will make professional judgment obsolete. However, the most successful applications show that AI works best as a tool that enhances human expertise. Radiologists and physicists are still the final decision-makers, but they are now armed with better, more detailed information. AI can automate the repetitive, data-heavy parts of quality control, which frees up skilled professionals to focus on complex problem-solving and improving clinical protocols.
This cooperative relationship is also seen in the reporting process itself. The accuracy of the final radiology report is a critical part of quality control. This importance is reflected in the growing market for radiology report quality assurance services, which was valued at around USD 63.4 million in 2024 and is projected to nearly double to USD 113.5 million by 2034. You can find more details about these market trends in this report on radiology report quality assurance services.
Beyond the imaging process, AI also helps improve the quality of medical documentation. For example, exploring advancements in medical speech-to-text technology can make reporting faster and more accurate, which is vital for both patient care and quality control. This combination of AI in both image acquisition and reporting creates a more solid, end-to-end quality framework. The future of quality control in radiology isn’t about choosing between human oversight and automation, but about skillfully blending them to create safer, more reliable diagnostic pathways.
2. Navigating Regulations Without Losing Your Mind
Diving into the regulatory side of quality control in radiology can feel like trying to decipher a foreign language. It's a dense network of rules from federal agencies, state health departments, and professional accreditation bodies. But these rules aren't just red tape; they are the essential traffic laws for medical imaging, designed to make sure every facility operates safely and effectively. The key is to view compliance not as a burden, but as a foundational element of patient care.
The regulatory structure is layered. At the top, the U.S. Food and Drug Administration (FDA) establishes performance standards for all radiation-emitting equipment. Beneath that, individual state health departments often add more specific requirements for staff qualifications and testing schedules. Finally, accreditation bodies like the American College of Radiology (ACR) introduce their own strict criteria that frequently go beyond government mandates. For instance, the ACR might require more frequent phantom testing for a mammography unit than the state requires.
The Major Players in QC Regulation
To navigate this system, you need to know who sets the rules and what they prioritize. Think of it like driving: there are national highway laws, state speed limits, and even rules for a private community—all apply, but in different contexts. A well-run facility doesn't just follow the rules; it understands the purpose behind them.
- Federal Agencies (e.g., FDA): These organizations focus on equipment safety and manufacturing standards. Their role is to ensure that devices like X-ray machines and CT scanners are built to perform safely and reliably from the start.
- State Health Departments: States are generally responsible for licensing facilities and personnel. They oversee day-to-day operational safety, such as radiation shielding requirements and dose monitoring for staff.
- Accreditation Organizations (e.g., The Joint Commission, ACR): These are private, non-governmental groups that offer a seal of quality. Accreditation is often a condition for insurance reimbursement and shows a commitment to higher standards that surpass basic legal compliance.
To better understand their roles, here is a breakdown of some key regulatory bodies and their specific requirements.
| Regulatory Body | Scope of Authority | Key QC Requirements | Inspection Frequency |
|---|---|---|---|
| U.S. Food and Drug Administration (FDA) | Nationwide; focuses on equipment manufacturing and performance standards. | Sets baseline standards for new imaging equipment (e.g., mammography under MQSA). Mandates that facilities establish and maintain a quality assurance program. | Varies; often tied to specific programs like the Mammography Quality Standards Act (MQSA), which requires annual inspections. |
| American College of Radiology (ACR) | Voluntary accreditation organization; sets standards for best practices. | Modality-specific phantom testing, image quality reviews, peer review programs, and annual medical physicist surveys. Often more stringent than federal or state laws. | Every 3 years for accreditation renewal, which includes submission of clinical and phantom images. |
| The Joint Commission | Accredits and certifies healthcare organizations and programs nationwide. | Requires evidence of a comprehensive quality management program, regular equipment performance evaluation, radiation safety protocols, and staff competency assessments. | Unannounced on-site surveys occur at least every 39 months. |
| State Health Departments | Varies by state; licenses facilities and personnel within state borders. | Specifics vary widely but typically include equipment registration, shielding integrity tests, personnel dosimetry, and mandated QC test frequencies. | Typically ranges from annually to once every 3-5 years, depending on the state and type of facility. |
| Table: Key Regulatory Bodies and Their QC Requirements | |||
| Overview of major regulatory organizations and their specific quality control requirements for radiology facilities |
This table highlights that while there is overlap, each body has a distinct focus, from the FDA's equipment-centric view to the ACR's emphasis on peer-reviewed quality. A successful QC program integrates all these requirements into a single, cohesive strategy.
The image below, from the FDA's website, shows their guidance on establishing a quality assurance program, underscoring its importance.
This document stresses that a QC program should be both practical and effective, with the dual goals of maintaining high-quality diagnostic images while minimizing patient and staff radiation exposure.
Staying Compliant Without the Headache
Maintaining compliance can be challenging, especially during equipment upgrades, staff changes, or periods of growth. A common mistake is allowing documentation to fall behind actual practice. The best approach is to build a QC program that is both thorough and easy to manage. This involves creating clear, accessible documentation systems and providing ongoing staff training.
Navigating the dense landscape of healthcare regulations is vital for radiology quality control. New tools, like AI agents for healthcare policy analysis, can simplify this task by helping to interpret complex regulatory documents. By weaving compliance into the daily workflow, facilities can not only pass inspections but also deliver the safest, highest-quality care to every patient.
Building QC Programs That Actually Work
Creating a successful quality control in radiology program is like constructing a sturdy house—it needs a solid foundation, a clear blueprint, and careful work at every stage. It’s about moving beyond a QC manual that gathers dust on a shelf and building a living system that actively improves patient safety and diagnostic confidence. The process starts with an honest, detailed look at your current practices to create a practical roadmap for improvement.

Assessing Your Current QC Status
Before you can build something better, you need to know your starting point. This initial assessment goes deeper than just reading old inspection reports; it means taking a close look at your day-to-day operations. A great first step is to map out your existing QC workflows. Trace the journey of a patient image from the moment it's taken to the final report, identifying every quality checkpoint.
This self-audit helps you see both your strengths and weaknesses. For instance, your technologists might be great at daily phantom scans, but the documentation could be inconsistent. Or perhaps your annual physicist reports are thorough, but their recommendations aren't consistently put into action. Pinpointing these gaps is the first step toward a program that solves real problems, not just theoretical ones.
Key Elements of a Practical Program
Once you know where to improve, the next phase is to design a program with practical elements your team can realistically follow. A functional QC program stands on three pillars: effective training, helpful documentation, and meaningful performance tracking.
- Effective Staff Training: Training shouldn't be a one-off event during onboarding; it needs to be continuous. It should focus on the "why" behind each QC task, not just the "how." When a technologist understands that a specific check helps prevent unnecessary radiation exposure, they become more invested in doing it right.
- Helpful Documentation Systems: Documentation should be a useful tool, not a chore. The goal is to make it easy for staff to log results and simple for managers to review trends. If a system is so complicated that people avoid it, it's failing. Digital logbooks and simple checklists are often more effective than bulky binders.
- Meaningful Performance Tracking: Monitor key metrics that genuinely reflect quality. This could include repeat/reject rates, equipment uptime, or feedback from radiologists on image quality. Tracking these figures provides solid proof of progress and helps justify further investment in quality initiatives.
Overcoming Implementation Hurdles
Even the most well-designed plans can hit roadblocks. Common challenges include budget limitations, staff resistance to change, and the difficulty of keeping the momentum going. When building dependable QC programs, it's vital to think about the physical setup. For example, ensuring reliable medical workstation and mounting solutions are in place is a foundational step for operational quality in healthcare facilities.
To get everyone on board, you have to communicate the benefits clearly. Position the changes not as extra work, but as a way to improve patient outcomes and make everyone's job easier. Celebrate small wins—like a month with a record-low image rejection rate—to keep the team motivated and demonstrate that their efforts are making a real difference. This approach turns quality control from a top-down rule into a shared goal of excellence.
What's Next for Quality Control in Radiology
The path forward for quality control in radiology involves a blend of intelligent technology, flexible regulations, and a renewed emphasis on professional development. As imaging devices grow more powerful, the quality control methods we rely on must also advance. This is more than just buying new software; it's about creating QC frameworks that can handle future innovations without risking patient safety. The aim is to build systems that are not only effective now but are also prepared for what's to come.
The Evolution of Intelligent Quality Monitoring
Artificial intelligence is poised to do much more than its current tasks. The next wave of AI in quality control will act less like a simple alarm and more like a collaborative learning system. Think of an AI that doesn't just flag one subpar image but instead reviews thousands of scans to spot a CT scanner's slow, subtle performance decline over time. These future systems will offer adaptive quality monitoring, learning the specific performance patterns of each machine and automatically tweaking its monitoring criteria.
This smart oversight will help facilities predict maintenance needs with much better accuracy. For instance, by studying a vast dataset of scans, an AI might link minor image artifacts to specific environmental conditions, like shifts in room temperature, leading to precise operational adjustments.
Preparing for a New Era of QC
To stay current in this dynamic field, a dedication to ongoing learning is essential. As technology advances, so must the skills of the professionals who use it. This means radiologists and technicians will need continuous education not only on new imaging methods but also on the QC protocols that ensure their reliability.
To develop a program ready for the future, you can follow these strategies:
- Invest in Continuous Education: Set aside resources for your team to attend workshops and training sessions on new QC technologies and standards.
- Foster a Culture of Adaptability: Motivate your team to welcome new workflows and technologies, presenting them as chances for growth and improvement.
- Engage with Innovators: Keep up with new AI tools and regulatory changes by following industry publications and joining professional forums.
The future of quality control is proactive, not just reactive. Companies are compiling huge datasets to train AI that will improve diagnostic accuracy and deepen our understanding of health. By adopting these advancements and getting teams ready for change, healthcare facilities can make sure their QC programs stay effective and continue to safeguard patients for years to come.
At PYCAD, we create the AI solutions that will drive this next stage of medical imaging. Our background in data management and model implementation helps medical device companies put intelligent quality control right into their products, improving both diagnostic precision and operational workflow. Find out how we can help you shape the future of medical imaging by visiting pycad.co.