Think of your medical device not just as an innovative piece of technology, but as a promise. A promise of safety, reliability, and effectiveness to every single patient who will depend on it. A Medical Device Quality Management System (QMS) is the architecture of that promise.
This isn't about ticking boxes on a bureaucratic checklist. It's the strategic engine that drives true innovation and builds the kind of market trust that lasts.
From Mandate to Mission: The Heart of Your QMS
Let's use an analogy. You wouldn't build a world-class race car by just throwing parts together and hoping it stays on the track. You'd follow a meticulous blueprint, making sure every component—from the engine to the brakes—works in perfect harmony. The goal isn't just to cross the finish line; it's to guarantee peak performance and prevent disaster.
Your QMS is that blueprint for your medical device.

More Than Just a Regulatory Hurdle
A well-designed QMS provides the structured framework for everything from initial design controls to post-market surveillance, ensuring your device performs flawlessly every time. It’s a powerful shift in perspective that transforms regulatory hurdles into opportunities to achieve excellence. This system empowers you to move beyond simply meeting the standards and start setting them, turning a compliance mandate into a core part of your mission.
The market is clearly showing this is non-negotiable. The global Medical Device QMS market is seeing explosive growth, with projections suggesting it could reach $3.0 billion or more by 2033. This surge is a direct response to increasingly stringent regulations from global bodies like the FDA and EMA. You can find more insights about this growing market demand on datainsightsmarket.com.
Building a Bedrock of Trust and Excellence
When you truly commit to a QMS, the benefits ripple out far beyond getting regulatory approval. It cultivates a deep-rooted culture of quality that touches every part of your organization.
This leads to some powerful outcomes:
- Enhanced Patient Safety: By systematically finding and neutralizing risks, your QMS becomes a direct contributor to better patient outcomes. It’s your first line of defense.
- Improved Product Efficacy: Rigorous controls mean your device does what it’s supposed to do, consistently. This is how you build unshakable credibility with clinicians.
- Streamlined Operational Efficiency: When processes are standardized, you naturally reduce errors, cut down on waste, and can even speed up your development timelines.
- Wider Market Access: A compliant QMS is your passport to the global stage, making it far simpler to enter new and lucrative regions.
At PYCAD, we live and breathe quality, especially when it comes to the complexities of medical imaging. We build custom web DICOM viewers and integrate them into sophisticated medical imaging platforms, always with a sharp focus on regulatory demands and data integrity. Our work, which you can see in our extensive portfolio, is a testament to how robust engineering and a quality-first mindset create solutions people can depend on.
This guide will walk you through how a strong QMS becomes your greatest asset, giving you the confidence to deliver life-changing technology to the world.
Navigating the Global Regulatory Landscape
Taking a medical device to the global market is a lot like navigating a ship through a vast, uncharted archipelago. Every new country is a new island, each with its own unique currents, customs, and rules of engagement. In this journey, your medical device quality management system isn't just a compliance document—it's your map, your compass, and your ship's log all rolled into one.
This isn't about memorizing dry legal text. It's about getting to the heart of why these regulations exist in the first place: patient safety. Once you grasp the fundamental mission behind the rules, you can transform them from intimidating hurdles into a clear, actionable playbook for success.
The Foundational Standards: ISO 13485 and 21 CFR 820
At the core of global medical device compliance, you'll find two foundational pillars. First, there's ISO 13485, the international gold standard for quality management systems in our industry. Think of it as the universal language of quality, a framework that’s respected and understood from Toronto to Tokyo.
The second pillar has long been the U.S. FDA's 21 CFR Part 820, also known as the Quality System Regulation (QSR). For decades, these were the specific rules of the road for any device sold in the United States. While it’s always been distinct from ISO 13485, a massive shift is happening right now. It's critical to understand the nuances of the FDA Approval Process for Medical Devices, as this process is deeply intertwined with your quality system.
The game-changer? The FDA's new Quality Management System Regulation (QMSR), which officially takes effect in February 2026. This new rule harmonizes the old QSR by directly incorporating ISO 13485. This is a huge deal. It signals a powerful move toward a more unified approach to compliance, making it far more straightforward for innovators to meet requirements in multiple markets—especially important when the U.S. makes up over 40% of the global medical device market.
To help you see how these pieces fit together, let’s break them down.
Key Regulatory Frameworks at a Glance
Navigating the global landscape means understanding the subtle but important differences between major regulatory bodies. The table below offers a high-level comparison of the key frameworks you'll encounter on your journey.
| Regulatory Framework | Governing Body or Region | Core Focus | Key Requirement Example |
|---|---|---|---|
| ISO 13485:2016 | International (ISO) | A process-based framework for a comprehensive QMS, emphasizing risk management throughout the product lifecycle. | Documented procedures for design and development controls, including verification and validation. |
| FDA 21 CFR Part 820 / QMSR | United States (FDA) | Ensuring the safety and effectiveness of devices marketed in the U.S. Now harmonized with ISO 13485. | Specific requirements for Corrective and Preventive Actions (CAPA) and complaint handling. |
| EU Medical Device Regulation (MDR) | European Union | A lifecycle approach with a heavy emphasis on clinical evidence, post-market surveillance, and transparency. | Maintaining a robust Post-Market Surveillance (PMS) plan and generating periodic safety update reports (PSURs). |
This snapshot shows that while each framework has its unique flavor, they all share the same goal: ensuring every device is safe, effective, and built on a foundation of quality.
Expanding Horizons: The EU MDR
As you chart your course beyond the U.S., you’ll inevitably sail into the waters of the European Union's Medical Device Regulation (MDR). This isn't just a minor update; the MDR represents a fundamental modernization of Europe's requirements, placing a much stronger emphasis on a total lifecycle approach to safety and quality.
Key areas of focus under the EU MDR include:
- Greater Clinical Evidence: The bar has been raised. The MDR demands more robust clinical data to prove a device's safety and performance claims before it can even touch the market.
- Enhanced Post-Market Surveillance (PMS): It’s no longer good enough to just react to problems. The regulation mandates a proactive, living system for continuously monitoring how your device performs in the real world.
- Increased Transparency: A central European database, EUDAMED, is being rolled out to provide unprecedented transparency and traceability for every device sold in the EU.
For any innovator with global ambitions, understanding these distinct yet overlapping frameworks is non-negotiable. You might find it helpful to dive deeper into navigating the medical device regulatory pathway, as a solid grasp of these rules is the bedrock of your entire market strategy.
A well-structured medical device quality management system doesn't just satisfy one regulator; it anticipates the demands of many. It builds a universal framework of quality that can be adapted to meet specific regional requirements, turning compliance into a competitive advantage.
The Core Pillars of a World-Class QMS
A truly great Quality Management System isn't some rigid, off-the-shelf program. Think of it more like a beautifully engineered structure, held up by a set of foundational pillars. Each one is critical, and they all work together to support your medical device—from the first napkin sketch to its final day in the field.
These pillars create a living, breathing system where quality isn't just an afterthought checked at the finish line. It's woven into the very fabric of how you operate. Let’s walk through each of these core pillars and see how they lock together to build an unshakable foundation for your work.
Design Controls: Building Quality in from Day One
You can't inspect quality into a medical device. You have to design it in from the very start. That’s the entire philosophy behind Design Controls. This is your architectural blueprint, where every choice is deliberately planned, documented, and challenged.
It’s a disciplined process that ensures the needs of the patient and doctor are translated into clear design inputs (your requirements). Those requirements are then systematically built into design outputs (the actual specifications and drawings). Every step is double-checked through verification and validation, answering two critical questions: Did we build the device correctly? And did we build the correct device?
Risk Management: Getting Ahead of What Could Go Wrong
While design controls map out what your device should do, Risk Management is all about what could go wrong. This pillar is about getting out in front of potential problems and systematically identifying, evaluating, and neutralizing hazards before they have any chance of harming a patient. It’s the safety engineering that runs in parallel with every other activity.
A world-class QMS treats risk management not as a checkbox exercise, but as a continuous conversation. It asks "what if?" at every stage, from initial design to post-market feedback, ensuring patient safety remains the ultimate priority.
Effective risk management isn't about guesswork; it's a structured approach to analyzing potential failures, understanding their severity, and putting controls in place to bring those risks down to an acceptable level. To really get into the weeds on this, check out our deep dive in our guide on medical device risk management, where we break it all down.
Document Control: Your Single Source of Truth
Imagine trying to assemble a passenger jet with different teams using different, outdated versions of the blueprints. It would be an absolute disaster. Document and Record Control is the pillar that prevents that kind of chaos by creating one undisputed source of truth for your entire operation.
This system governs how every critical document—from your Standard Operating Procedures (SOPs) to detailed design specs—is written, reviewed, approved, and updated. It guarantees everyone is working from the same, most current playbook, creating the consistency and traceability that regulators demand.
Supplier Management: Extending Quality Beyond Your Four Walls
Let’s be honest, modern medical devices are rarely built entirely in-house. You rely on a whole network of suppliers for critical components, materials, and services. Supplier Management is the pillar that extends your quality standards to every single partner in your supply chain.
This means:
- Thorough Vetting: You don't just pick the cheapest option. You evaluate and select suppliers based on their proven ability to meet your quality and regulatory standards.
- Clear Agreements: You establish rock-solid quality agreements that leave no room for ambiguity, defining expectations, responsibilities, and specs.
- Ongoing Monitoring: You keep a close eye on supplier performance through audits, inspections, and clear metrics to ensure they never slip.
Remember, your device is only as strong as its weakest link. Great supplier management ensures every link in that chain is rock-solid.
CAPA: Turning Mistakes into Momentum
Look, no process is perfect. Mistakes are going to happen. The true test of your QMS is how you respond. Corrective and Preventive Action (CAPA) is your formal process for digging into problems, fixing them, and—most importantly—making sure they never happen again.
A corrective action puts out a fire that has already started. A preventive action spots the faulty wiring before the fire can even begin. This is the pillar that transforms your QMS from a static rulebook into a dynamic, learning system that gets smarter over time.
Post-Market Surveillance: Listening to the Real World
Once your device is out helping patients, your job is far from over. Post-Market Surveillance (PMS) is the pillar that keeps you connected to the real world by actively collecting and analyzing data on how your device is actually performing.
This feedback loop is where the magic happens. A patient complaint from your PMS system can trigger a CAPA. That CAPA investigation might uncover a hidden design flaw, which then feeds back into your design controls for the next product iteration. Every step is documented, creating a closed-loop system that listens, learns, and adapts.
This holistic approach is absolutely critical, yet a survey of MedTech professionals found that a staggering 69% lack confidence that their QMS can scale with their company's growth. Many reported spending over 50 hours a month just on reactive fixes. You can discover more insights about these quality challenges and see exactly why building on these strong pillars is the only way to succeed long-term.
Your Step-by-Step QMS Implementation Roadmap
Building a quality management system for a medical device isn't a race to some imaginary finish line. It's a thoughtful, deliberate process of laying a foundation that will support your company for years to come. If you try to do everything at once, you'll just end up overwhelmed.
Think of it as a four-phase journey. Each stage builds on the one before it, breaking down a massive undertaking into a series of clear, manageable steps. This roadmap is designed to help you start lean—especially crucial for startups—and then scale your QMS as your company and product line grow.
Phase 1: Foundation and Planning
This is where it all begins. Before you write a single procedure, you need to define the very heart and soul of your QMS. It all starts with your Quality Policy. This isn't just some fluffy mission statement; it's a direct, powerful commitment from the top brass to quality, safety, and always getting better. It’s your North Star.
Next, you have to define the scope. You need to get specific and ask some tough questions:
- Which exact products will this QMS cover?
- What are the core processes involved, from the first sketch to final delivery?
- Are there any parts of ISO 13485 that genuinely don't apply to how you operate? (Be careful here!)
Nailing this foundation means every single thing you do from this point forward will be aligned. It stops you from wasting time and keeps the whole team laser-focused on what actually matters.
This diagram shows how the core pillars of a QMS flow into one another, guiding you through the whole process.
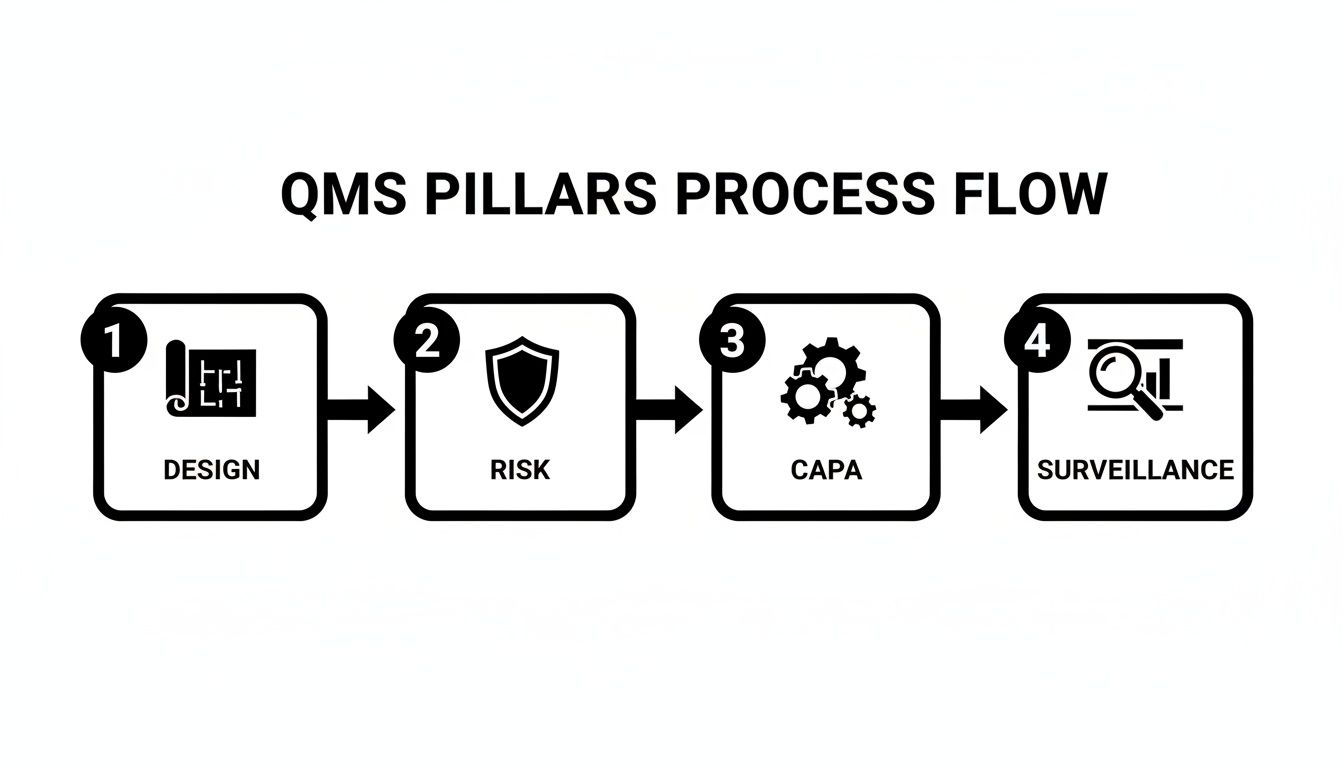
You can see how everything starts with a solid design, is shielded by smart risk management, gets fixed when things go wrong through CAPA, and is constantly refined by keeping an eye on the market.
Phase 2: Process Development and Documentation
With the foundation poured, it's time to erect the framework. This phase is all about turning your quality policy into action by creating clear processes and documenting them. This is where you write your Standard Operating Procedures (SOPs)—the playbooks your team will live by for every critical activity.
Your SOPs are basically the DNA of your quality system. They need to be sharp, clear, and practical. Don’t try to write them all at once. Take a risk-based approach and start with the big ones first. For a startup, that usually means design controls, risk management, and document control.
I see this mistake all the time: companies over-document way too early. A QMS for a startup should be agile and lean, not buried under a mountain of paperwork. The goal is to create procedures that are tough enough to be compliant but flexible enough to grow with you.
Phase 3: Implementation and Training
A QMS on paper is just a stack of documents. This is the phase where you breathe life into it. You'll start rolling out your new procedures across the company, but more importantly, you have to make sure every single person understands their part in the system.
Training is everything here. It's not enough to just email an SOP and call it a day. You have to ensure people get the "why" behind the "what." When training is done right, your team stops being passive followers and becomes active owners of quality. That’s how you get your QMS principles to stick.
At PYCAD, we live and breathe this stuff. We build custom web DICOM viewers and integrate them into medical imaging web platforms, which means quality and compliance have to be woven into the very fabric of our work from day one. You can see how we apply these rigorous principles in our own work on our portfolio page.
Phase 4: Monitoring and Improvement
Finally, remember that your QMS is a living, breathing thing—not a dusty binder on a shelf. This last phase is all about creating the feedback loops that fuel continuous improvement. You're checking to see if the system is actually working and actively hunting for ways to make it better.
Two key activities drive this phase:
- Internal Audits: Think of these as routine health check-ups for your QMS. You systematically review your own processes against your SOPs and the regulations to find any gaps or issues before an external auditor does.
- Management Reviews: This is where the leadership team steps back to look at the big picture. By reviewing data from audits, customer complaints, and process metrics, they can make smart, strategic decisions to ensure the QMS is still doing its job and helping the business succeed.
This four-phase approach takes the mystery out of QMS implementation. It gives you a clear path to building a system that doesn't just check a box for regulators but actually becomes a powerful engine for innovation and growth.
Weaving Quality into Digital Health and SaMD
When your medical device is the code, the old rulebook for quality management gets a serious update. We're in a new era where software isn't just part of a device—it's the whole show. For the world of Software as a Medical Device (SaMD), AI-powered diagnostics, and connected health platforms, the quality challenges are completely different from those of traditional hardware.

Here, quality isn't about the precision of a manufactured part. It’s about the integrity of an algorithm, the security of patient data, and the flawless flow of information. The core mission—patient safety and device effectiveness—is the same, but getting there requires a fundamentally new approach.
Your North Star for Software: IEC 62304
For anyone building SaMD, the international standard IEC 62304 is your essential guide. It provides a risk-based framework for the entire software development lifecycle, making sure that quality and safety are designed into the product from the very first line of code, not just inspected at the end.
This standard instills a disciplined approach that covers:
- Software Development Planning: Laying out a detailed roadmap before you even think about coding.
- Requirements Analysis: Clearly defining, in testable terms, exactly what the software needs to accomplish.
- Architectural Design: Building a software structure that is strong, secure, and easy to maintain down the line.
- Verification and Validation: Putting the software through its paces to confirm it was built right and actually solves the user's problem.
Following this structured process is non-negotiable for SaMD. We strongly recommend diving deeper into this topic with our guide on medical device software validation to see how to put these principles into action.
The Unique Hurdles of AI and Medical Imaging
Bringing artificial intelligence into the mix adds another fascinating layer of complexity. An AI diagnostic tool isn't a static piece of software; it can learn and change. Your QMS has to be nimble enough to handle algorithmic updates, maintain perfect traceability for machine learning models, and validate the datasets used for training.
This is especially critical in medical imaging. The integrity of complex data formats like DICOM is everything. A single corrupted file or a tiny flaw in how an image is displayed could lead to a catastrophic misdiagnosis. This is where solid, validated integrations become the foundation of quality.
In the SaMD world, your Quality Management System isn't a dusty binder of procedures. It's a living, breathing framework that governs the entire data journey—from acquisition and processing to secure storage and diagnostic display.
At PYCAD, this is precisely our wheelhouse. We build custom web DICOM viewers and integrate them into medical imaging web platforms. Our entire process is grounded in the principles of IEC 62304, so our solutions are built from the ground up to integrate seamlessly into a compliant medical device quality management system. You can see how we build these robust, quality-first systems over on our portfolio page.
Cybersecurity and Data Integrity: The New Bedrock of Quality
Finally, for any connected medical device, cybersecurity is no longer just an IT problem—it’s a patient safety imperative. A modern QMS must include a proactive cybersecurity risk management plan that anticipates vulnerabilities and puts controls in place to shield both the device and sensitive patient data. This is a particular challenge for fast-moving digital health tools like portable electrocardiogram monitors, which merge hardware, software, and data processing in real time.
Data integrity is the other side of that coin. Your system must absolutely guarantee that data remains accurate, complete, and untampered with from start to finish. This means rigorous access controls, detailed audit trails, and secure data transfer protocols. For SaMD, quality and security are two sides of the same coin, and they are the pillars upon which patient trust is built.
Your Top Questions About Medical Device QMS, Answered
Jumping into medical device development is an incredible journey, but it’s natural to have a lot of questions. Building a quality management system that is both effective and compliant can feel like a mountain to climb. Let's tackle some of the most common questions innovators ask, so you can move forward with confidence.
What’s the Real Cost of Implementing a QMS?
There’s no single price tag for a QMS. The investment really depends on your company’s size, how complex your device is, and the path you choose—whether that's a manual, paper-based system or a specialized electronic QMS (eQMS).
A lean startup might get by with a few thousand dollars for solid templates and some expert guidance. On the other hand, an established company could easily invest over $50,000 a year for a powerful eQMS software subscription. The key is to stop thinking of it as a cost and see it for what it is: a strategic investment. A well-built QMS is your best defense against cripplingly expensive recalls and your fastest route to market.
Can’t I Just Use Jira or Confluence for My QMS?
I get this question all the time. While tools like Jira and Confluence are fantastic for managing projects, they simply weren't designed for the strict regulatory world of medical devices. They're missing the built-in guardrails needed for compliance with standards like FDA 21 CFR Part 11, which governs electronic records and signatures.
Purpose-built eQMS platforms are a game-changer. They come with pre-validated modules for your most critical processes—design controls, risk management, and CAPA. This dramatically lowers your compliance risk and lifts the massive burden of having to validate the entire system from scratch yourself.
When Should a Startup Actually Build a QMS?
The best time to start was yesterday. The next best time is right now. You don’t need a perfect, all-encompassing system from day one, but weaving quality principles into your work from the very beginning is non-negotiable.
Focus on the fundamentals first, like design controls and risk management. It is infinitely easier to document your journey as you go than to scramble and try to piece together the evidence for regulators months or years later. The secret is to start lean, but always, always start early.
At PYCAD, we know firsthand that a solid QMS is the foundation for any safe and successful medical technology. It’s why we build quality and compliance into every custom web DICOM viewer and medical imaging platform we create. Want to see how we help turn brilliant ideas into market-ready realities? Take a look at our work on our portfolio page.






